NIH Public Access Author Manuscript Anticancer Agents Med Chem
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shilin Yang Doctor of Philosophy
PHYTOCHEMICAL STUDIES OF ARTEMISIA ANNUA L. THESIS Presented by SHILIN YANG For the Degree of DOCTOR OF PHILOSOPHY of the UNIVERSITY OF LONDON DEPARTMENT OF PHARMACOGNOSY THE SCHOOL OF PHARMACY THE UNIVERSITY OF LONDON BRUNSWICK SQUARE, LONDON WC1N 1AX ProQuest Number: U063742 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest U063742 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 ACKNOWLEDGEMENT I wish to express my sincere gratitude to Professor J.D. Phillipson and Dr. M.J.O’Neill for their supervision throughout the course of studies. I would especially like to thank Dr. M.F.Roberts for her great help. I like to thank Dr. K.C.S.C.Liu and B.C.Homeyer for their great help. My sincere thanks to Mrs.J.B.Hallsworth for her help. I am very grateful to the staff of the MS Spectroscopy Unit and NMR Unit of the School of Pharmacy, and the staff of the NMR Unit, King’s College, University of London, for running the MS and NMR spectra. -

Analysis of the Binding and Interaction Patterns of 100 Flavonoids with the Pneumococcal Virulent Protein Pneumolysin: an in Silico Virtual Screening Approach
Available online a t www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2016, 8 (16):40-51 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4 Analysis of the binding and interaction patterns of 100 flavonoids with the Pneumococcal virulent protein pneumolysin: An in silico virtual screening approach Udhaya Lavinya B., Manisha P., Sangeetha N., Premkumar N., Asha Devi S., Gunaseelan D. and Sabina E. P.* 1School of Biosciences and Technology, VIT University, Vellore - 632014, Tamilnadu, India 2Department of Computer Science, College of Computer Science & Information Systems, JAZAN University, JAZAN-82822-6694, Kingdom of Saudi Arabia. _____________________________________________________________________________________________ ABSTRACT Pneumococcal infection is one of the major causes of morbidity and mortality among children below 2 years of age in under-developed countries. Current study involves the screening and identification of potent inhibitors of the pneumococcal virulence factor pneumolysin. About 100 flavonoids were chosen from scientific literature and docked with pnuemolysin (PDB Id.: 4QQA) using Patch Dockprogram for molecular docking. The results obtained were analysed and the docked structures visualized using LigPlus software. It was found that flavonoids amurensin, diosmin, robinin, rutin, sophoroflavonoloside, spiraeoside and icariin had hydrogen bond interactions with the receptor protein pneumolysin (4QQA). Among others, robinin had the highest score (7710) revealing that it had the best geometrical fit to the receptor molecule forming 12 hydrogen bonds ranging from 0.8-3.3 Å. Keywords : Pneumococci, pneumolysin, flavonoids, antimicrobial, virtual screening _____________________________________________________________________________________________ INTRODUCTION Streptococcus pneumoniae is a gram positive pathogenic bacterium causing opportunistic infections that may be life-threating[1]. Pneumococcus is the causative agent of pneumonia and is the most common agent causing meningitis. -

Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney
antioxidants Review Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney Daniel Muñoz-Reyes 1,2,3 , Ana I. Morales 1,2,3,4,5,* and Marta Prieto 1,2,3,4,5 1 Toxicology Unit, University of Salamanca, 37007 Salamanca, Spain; [email protected] (D.M.-R.); [email protected] (M.P.) 2 Department of Physiology and Pharmacology, University of Salamanca, 37007 Salamanca, Spain 3 Group of Translational Research on Renal and Cardiovascular Diseases (TRECARD), 37007 Salamanca, Spain 4 Institute of Biomedical Research of Salamanca (IBSAL), 37007 Salamanca, Spain 5 National Network for Kidney Research REDINREN, RD016/0009/0025, Instituto de Salud Carlos III, 28029 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-677-555-055 Abstract: Quercetin is a flavonoid with antioxidant, antiviral, antimicrobial, and anti-inflammatory properties. Therefore, it has been postulated as a molecule with great therapeutic potential. The renoprotective capacity of quercetin against various toxins that produce oxidative stress, in both in vivo and in vitro models, has been shown. However, it is not clear whether quercetin itself or any of its metabolites are responsible for the protective effects on the kidney. Although the pharmacokinetics of quercetin have been widely studied and the complexity of its transit throughout the body is well known, the metabolic processes that occur in the kidney are less known. Because of that, the objective of this review was to delve into the molecular and cellular events triggered Citation: Muñoz-Reyes, D.; Morales, by quercetin and/or its metabolites in the tubular cells, which could explain some of the protective A.I.; Prieto, M. -

Type of the Paper (Article
foods Article Sustainable Extraction Techniques for Obtaining Antioxidant and Anti-Inflammatory Compounds from the Lamiaceae and Asteraceae Species Marisol Villalva 1 , Susana Santoyo 1 , Lilia Salas-Pérez 2, María de las Nieves Siles-Sánchez 1 , Mónica Rodríguez García-Risco 1, Tiziana Fornari 1, Guillermo Reglero 1,3 and Laura Jaime 1,* 1 Institute of Food Science Research (CIAL), Universidad Autónoma de Madrid (CEI UAM+CSIC), 28049 Madrid, Spain; [email protected] (M.V.); [email protected] (S.S.); [email protected] (M.d.l.N.S.-S.); [email protected] (M.R.G.-R.); [email protected] (T.F.); [email protected] (G.R.) 2 Faculty of Accounting and Administration, Universidad Autónoma de Coahuila, Fco. Javier Mina 150, Luis Echeverría Álvarez Sector Norte, 27085 Torreón, Coahuila, Mexico; [email protected] 3 Imdea-Food Institute, Universidad Autónoma de Madrid (CEI UAM+CSIC), 28049 Madrid, Spain * Correspondence: [email protected]; Tel.: +34-910-017-925 Abstract: Melissa officinalis L. and Origanum majorana L., within Lamiaceae family, and Calendula officinalis L. and Achillea millefolium L., within the Asteraceae, have been considered a good source of bioactive ingredients with health benefits. In this study, the supercritical fluid extraction (SFE) using pure CO2, and the ultrasound assisted extraction (UAE) were proposed as green techniques Citation: Villalva, M.; Santoyo, S.; to obtain plant-based extracts with potential antioxidant and anti-inflammatory activities. Higher Salas-Pérez, L.; Siles-Sánchez, values of total phenolic content and antioxidant activity were achieved in UAE ethanol:water (50:50, M.d.l.N.; Rodríguez García-Risco, M.; v/v) extracts. -

Rhamnus Alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties
antioxidants Review Rhamnus alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties Amine Nekkaa 1,2,*, Akila Benaissa 3, Fabrice Mutelet 2 and Laetitia Canabady-Rochelle 2,* 1 Process Engineering Laboratory for Sustainable Development and Health Products, Department of Process Engineering, National Polytechnic School of Constantine—Malek Bennabi, Constantine 25000, Algeria 2 Laboratoire Réactions et Génie des Procédés, CNRS, Université de Lorraine, F-54000 Nancy, France; [email protected] 3 Laboratory of Process Engineering for the Environment (LIPE), Department of Pharmaceutical Engineering, Faculty of Process Engineering, Salah Boubnider University, Constantine 3, Constantine 25000, Algeria; [email protected] * Correspondence: [email protected] (A.N.); [email protected] (L.C.-R.) Abstract: Rhamnus alaternus, is a wild-growing shrub, belonging to the Rhamnaceae family. Widely distributed in the Mediterranean basin, R. alaternus is used in the usual medicine in numerous countries, mostly Tunisia, Algeria, Morocco, Spain, France, Italy, and Croatia. A large number of disorders—including dermatological complications, diabetes, hepatitis, and goiter problems— can be treated by the various parts of R. alaternus (i.e., roots, bark, berries, and leaves). Several bioactive compounds were isolated from R. alaternus, including flavonoids, anthocyanins, and Citation: Nekkaa, A.; Benaissa, A.; anthraquinones, and showed several effects such as antioxidant, antihyperlipidemic, antigenotoxic, Mutelet, F.; Canabady-Rochelle, L. antimutagenic, antimicrobial, and antiproliferative. This review summarizes the updated information Rhamnus alaternus Plant: Extraction of concerning the botanical description, distribution, extraction processes applied on R. alaternus, and Bioactive Fractions and Evaluation of its ethnopharmacology, toxicity, phytochemistry, and pharmacological effects. -

Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,*
Chemodiversity of Exudate Flavonoids in Seven Tribes of Cichorioideae and Asteroideae (Asteraceae)§ Karin M.Valant-Vetscheraa and Eckhard Wollenweberb,* a Department of Plant Systematics and Evolution Ð Comparative and Ecological Phytochemistry, University of Vienna, Rennweg 14, A-1030 Wien, Austria b Institut für Botanik der TU Darmstadt, Schnittspahnstrasse 3, D-64287 Darmstadt, Germany. E-mail: [email protected] * Author for correspondence and reprint requests Z. Naturforsch. 62c, 155Ð163 (2007); received October 26/November 24, 2006 Members of several genera of Asteraceae, belonging to the tribes Mutisieae, Cardueae, Lactuceae (all subfamily Cichorioideae), and of Astereae, Senecioneae, Helenieae and Helian- theae (all subfamily Asteroideae) have been analyzed for chemodiversity of their exudate flavonoid profiles. The majority of structures found were flavones and flavonols, sometimes with 6- and/or 8-substitution, and with a varying degree of oxidation and methylation. Flava- nones were observed in exudates of some genera, and, in some cases, also flavonol- and flavone glycosides were detected. This was mostly the case when exudates were poor both in yield and chemical complexity. Structurally diverse profiles are found particularly within Astereae and Heliantheae. The tribes in the subfamily Cichorioideae exhibited less complex flavonoid profiles. Current results are compared to literature data, and botanical information is included on the studied taxa. Key words: Asteraceae, Exudates, Flavonoids Introduction comparison of accumulation trends in terms of The family of Asteraceae is distributed world- substitution patterns is more indicative for che- wide and comprises 17 tribes, of which Mutisieae, modiversity than single compounds. Cardueae, Lactuceae, Vernonieae, Liabeae, and Earlier, we have shown that some accumulation Arctoteae are grouped within subfamily Cichori- tendencies apparently exist in single tribes (Wol- oideae, whereas Inuleae, Plucheae, Gnaphalieae, lenweber and Valant-Vetschera, 1996). -

Study on Structure Activity Relationship of Natural Flavonoids
1 of 15 1 Study on Structure Activity Relationship of Natural 2 Flavonoids against Thrombin by Molecular Docking 3 Virtual Screening combined with Activity Evaluation 4 in vitro 5 Xiaoyan Wang1, 2 # , Zhen Yang3, 4 #, Feifei Su3, 4, Jin Li1, Evans Owusu Boadi 1, 2 , Yan-xu Chang1, 2*, 6 Hui Wang3, 4* 7 1 Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese 8 Medicine, Tianjin, 300193, China; 9 2 Tianjin Key Laboratory of Phytochemistry and Pharmaceutical Analysis, Tianjin University of Traditional 10 Chinese Medicine, Tianjin, 300193, China; 11 3 Tianjin Key Laboratory of Chinese medicine Pharmacology, Tianjin University of Traditional Chinese 12 Medicine, Tianjin, 300193, China 13 4 College of Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, Tianjin, 300193, 14 China 15 16 * Corresponding author: [email protected] (H.W.); [email protected] (Y.C) 17 Tel. /FAX: +86-22-59596163(S.F. &Y.C.) 18 # These authors contributed equally to this work. 19 20 21 22 Table S1 the docking score of 103 Compounds (In descending order of “-CDOCKER_energy”) 23 Figure S1 the Molecular structures of 42 flavonoids 24 25 26 27 28 Table S1 the docking score of 103 Compounds (In descending order of “-CDOCKER_energy”) 29 - -CDOCKER_ CDOCKER_IN energy TERACTION_ No. CAS compound (kcal/mol) energy (kcal/mol) 1 1257-08-5 (-)-Epicatechin gallate 52.0751 54.6293 2 83104-87-4 (-)-EGCG-3''-O-Me 51.2391 50.5546 3 20315-25-7 Proanthocyanidin B1 42.3509 55.9285 4 27200-12-0 Dihydromyricetin 40.3383 -

Quercetin and Its Derivatives: Chemical Structure and Bioactivity – a Review
POLISH JOURNAL OF FOOD AND NUTRITION SCIENCES www.pan.olsztyn.pl/journal/ Pol. J. Food Nutr. Sci. e-mail: [email protected] 2008, Vol. 58, No. 4, pp. 407-413 QUERCETIN AND ITS DERIVATIVES: CHEMICAL STRUCTURE AND BIOACTIVITY – A REVIEW Małgorzata Materska Research Group of Phytochemistry, Department of Chemistry, Agricultural University, Lublin Key words: quercetin, phenolic compounds, bioactivity Quercetin is one of the major dietary flavonoids belonging to a group of flavonols. It occurs mainly as glycosides, but other derivatives of quercetin have been identified as well. Attached substituents change the biochemical activity and bioavailability of molecules when compared to the aglycone. This paper reviews some of recent advances in quercetin derivatives according to physical, chemical and biological properties as well as their content in some plant derived food. INTRODUCTION of DNA synthesis, inhibition of cancerous cell growth, de- crease and modification of cellular signal transduction path- In recent years, nutritionists have shown an increased in- ways [Erkoc et al., 2003]. terest in plant antioxidants which could be used in unmodi- In food, quercetin occurs mainly in a bounded form, with fied form as natural food preservatives to replace synthetic sugars, phenolic acids, alcohols etc. After ingestion, deriva- substances [Kaur & Kapoor, 2001]. Plant extracts contain tives of quercetin are hydrolyzed mostly in the gastrointes- various antioxidant compounds which occur in many forms, tinal tract and then absorbed and metabolised [Scalbert thus offering an attractive alternative to chemical preserva- & Williamson, 2000; Walle, 2004; Wiczkowski & Piskuła, tives. A small intake of these compounds and their structural 2004]. Therefore, the content and form of all quercetin de- diversity minimize the risk of food allergies. -

Flavonoids from Artemisia Annua L. As Antioxidants and Their Potential Synergism with Artemisinin Against Malaria and Cancer
Molecules 2010, 15, 3135-3170; doi:10.3390/molecules15053135 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer 1, 2 3 4 Jorge F.S. Ferreira *, Devanand L. Luthria , Tomikazu Sasaki and Arne Heyerick 1 USDA-ARS, Appalachian Farming Systems Research Center, 1224 Airport Rd., Beaver, WV 25813, USA 2 USDA-ARS, Food Composition and Methods Development Lab, 10300 Baltimore Ave,. Bldg 161 BARC-East, Beltsville, MD 20705-2350, USA; E-Mail: [email protected] (D.L.L.) 3 Department of Chemistry, Box 351700, University of Washington, Seattle, WA 98195-1700, USA; E-Mail: [email protected] (T.S.) 4 Laboratory of Pharmacognosy and Phytochemistry, Ghent University, Harelbekestraat 72, B-9000 Ghent, Belgium; E-Mail: [email protected] (A.H.) * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 26 January 2010; in revised form: 8 April 2010 / Accepted: 19 April 2010 / Published: 29 April 2010 Abstract: Artemisia annua is currently the only commercial source of the sesquiterpene lactone artemisinin. Since artemisinin was discovered as the active component of A. annua in early 1970s, hundreds of papers have focused on the anti-parasitic effects of artemisinin and its semi-synthetic analogs dihydroartemisinin, artemether, arteether, and artesunate. Artemisinin per se has not been used in mainstream clinical practice due to its poor bioavailability when compared to its analogs. In the past decade, the work with artemisinin-based compounds has expanded to their anti-cancer properties. -

The Analysis of the Saltzman Collection of Peruvian Dyes by High
Armitage et al. Herit Sci (2019) 7:81 https://doi.org/10.1186/s40494-019-0319-1 RESEARCH ARTICLE Open Access The analysis of the Saltzman Collection of Peruvian dyes by high performance liquid chromatography and ambient ionisation mass spectrometry Ruth Ann Armitage1* , Daniel Fraser2, Ilaria Degano3 and Maria Perla Colombini3 Abstract Yarn samples from the Saltzman Collection of Peruvian dyes were characterized by several diferent analytical tech- niques: high performance liquid chromatography with both diode array detection (HPLC-DAD) and electrospray ionisation with tandem mass spectrometry (HPLC-ESI-Q-ToF), direct analysis in real time (DART) mass spectrometry and paper spray mass spectrometry. This report serves primarily as a database of chemical information about the col- orants in these dye materials for those studying ancient South American textiles and their colorants. We also provide a comparison of the results obtained by currently widespread HPLC techniques with those of two diferent ambient ionisation direct mass spectrometry methods to highlight the advantages and disadvantages of these approaches. Keywords: Natural dyes, HPLC, Ambient ionisation mass spectrometry, DART-MS, Peruvian dyes, Saltzman color collection Introduction Peru. One of the laboratory’s frst major projects in the Max Saltzman began his career in industrial color chem- 1970s resulted in the Saltzman Collection of Peruvian istry after the Second World War. From the 1960s, he dyes, a notebook containing recipes and descriptions of consulted with museums and researchers to identify materials collected and prepared by Saltzman. Te note- colorants in ancient textiles with the methods of the book, currently held in the collections at UCLA, also time, primarily solution ultraviolet–visible absorption contains skeins of wool (not specifed, but presumably spectroscopy. -
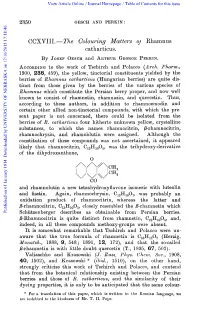
The Colouring Mattem OJ Rhamnus Catharticus
View Article Online / Journal Homepage / Table of Contents for this issue 2350 OESCH AND PERKIN: CCXVII1.-The Colouring Mattem OJ Rhamnus catharticus. By JOSEFOESCH and ARTHURGEORGE PERKIN. ACCORDINGto the work of Tschirch and Polacco (Arch. Yharm., 1900, 238, 459), tlie yellow, tinctorial constituents yielded by the berries of Bhamnus catharticus (Hungarian berries) are quite dis- tinct from those given by tlhe berries of the various species of Rhamnus which constitute the Persian berry proper, and now well known to consist of rhamnetin, rhamnazin, and quercetin. Thus, according to these authors, in addition to rhamnoemodin and certain other allied non-tinctorial compounds, with which the pre- sent paper is not concerned, there could be isolated from the berries of R. catharticus four hitherto unknown yellow, crystalline substances, to which the names rhamnocitrin, P-rhamncrcitrin, rhamnochrysin, and rhamnblutin were assigned. Although the constitution of these compounds was not ascertained, it appeared likely that rhamnocitrin, Cl3HI0O5, was the triliydroxy-derivative of the dihydroxanthone, 0 and rhamnolutin a new tetrahydroxyflavone isomeric with luteolin and fisetin. Again, rhamnochrysin, CI3Hl0O7, was probably an oxidation product of rhamnocitrin, whereas the latter and P-rhamnocitrin, C13H1006, closely resembled the P-rhamnetin which Published on 01 January 1914. Downloaded by UNIVERSITY OF NEBRASKA 17/10/2015 17:18:40. Schutzenberger describes as obtainable from Persian berries. 8-Rhamnocitrin is quite distinct from rhamnetin, C,,H,,O,, and, indeed, in all these compounds methoxy-groups were absent. It is somewhat remarkable that Tschirch and Polacco were un- aware that the true formula of rhamnetin is C16H120, (Herzig, Mosaartsh., 1888, 9, 548; 1891, 12, 172), and that the so-called P-rhamnetin is with little doubt quercetin (T., 1895, 67, 502). -

In Silico, in Vitro and in Vivo Memory Enhancing Activity
IN SILICO, IN VITRO AND IN VIVO MEMORY ENHANCING ACTIVITY OF CERTAIN COMMERCIALLY AVAILABLE FLAVONOIDS IN SCOPOLAMINE AND ALUMINIUM-INDUCED LEARNING IMPAIRMENT IN MICE Thesis submitted to The Tamil Nadu Dr. M.G.R. Medical University, Chennai for the award of the degree of DOCTOR OF PHILOSOPHY in PHARMACY Submitted by A. MADESWARAN, M. Pharm., Under the guidance of Dr. K. ASOK KUMAR, M. Pharm., Ph.D. College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore – 641 044, Tamil Nadu, India. JUNE 2017 Certificate This is to certify that the Ph.D. dissertation entitled “IN SILICO, IN VITRO AND IN VIVO MEMORY ENHANCING ACTIVITY OF CERTAIN COMMERCIALLY AVAILABLE FLAVONOIDS IN SCOPOLAMINE AND ALUMINIUM- INDUCED LEARNING IMPAIRMENT IN MICE” being submitted to The Tamil Nadu Dr. M.G.R. Medical University, Chennai, for the award of degree of DOCTOR OF PHILOSOPHY in the FACULTY OF PHARMACY was carried out by Mr. A. MADESWARAN, in College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore, under my direct supervision and guidance to my fullest satisfaction. The contents of this thesis, in full or in parts, have not been submitted to any other Institute or University for the award of any degree or diploma. Dr. K. Asok Kumar, M.Pharm., Ph.D. Professor & Head, Department of Pharmacology, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore, Tamil Nadu - 641 044. Place: Coimbatore – 44. Date: Certificate This is to certify that the Ph.D. dissertation entitled “IN SILICO, IN VITRO AND IN VIVO MEMORY ENHANCING ACTIVITY OF CERTAIN COMMERCIALLY AVAILABLE FLAVONOIDS IN SCOPOLAMINE AND ALUMINIUM- INDUCED LEARNING IMPAIRMENT IN MICE” being submitted to The Tamil Nadu Dr.