Important Progress Towards Elimination of Onchocerciasis in the West Region of Cameroon Guy-Roger Kamga1,2,3*, Fanny N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
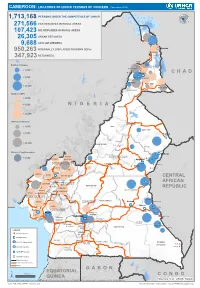
Page 1 C H a D N I G E R N I G E R I a G a B O N CENTRAL AFRICAN
CAMEROON: LOCATIONS OF UNHCR PERSONS OF CONCERN (November 2019) 1,713,168 PERSONS UNDER THE COMPETENCENIGER OF UNHCR 271,566 CAR REFUGEES IN RURAL AREAS 107,423 NIG REFUGEES IN RURAL AREAS 26,305 URBAN REFUGEES 9,688 ASYLUM SEEKERS 950,263 INTERNALLY DISPLACED PERSONS (IDPs) Kousseri LOGONE 347,923 RETURNEES ET CHARI Waza Limani Magdeme Number of refugees EXTRÊME-NORD MAYO SAVA < 3,000 Mora Mokolo Maroua CHAD > 5,000 Minawao DIAMARÉ MAYO TSANAGA MAYO KANI > 20,000 MAYO DANAY MAYO LOUTI Number of IDPs < 2,000 > 5,000 NIGERIA BÉNOUÉ > 20,000 Number of returnees NORD < 2,000 FARO MAYO REY > 5,000 Touboro > 20,000 FARO ET DÉO Beke chantier Ndip Beka VINA Number of asylum seekers Djohong DONGA < 5,000 ADAMAOUA Borgop MENCHUM MANTUNG Meiganga Ngam NORD-OUEST MAYO BANYO DJEREM Alhamdou MBÉRÉ BOYO Gbatoua BUI Kounde MEZAM MANYU MOMO NGO KETUNJIA CENTRAL Bamenda NOUN BAMBOUTOS AFRICAN LEBIALEM OUEST Gado Badzere MIFI MBAM ET KIM MENOUA KOUNG KHI REPUBLIC LOM ET DJEREM KOUPÉ HAUTS PLATEAUX NDIAN MANENGOUBA HAUT NKAM SUD-OUEST NDÉ Timangolo MOUNGO MBAM ET HAUTE SANAGA MEME Bertoua Mbombe Pana INOUBOU CENTRE Batouri NKAM Sandji Mbile Buéa LITTORAL KADEY Douala LEKIÉ MEFOU ET Lolo FAKO AFAMBA YAOUNDE Mbombate Yola SANAGA WOURI NYONG ET MARITIME MFOUMOU MFOUNDI NYONG EST Ngarissingo ET KÉLLÉ MEFOU ET HAUT NYONG AKONO Mboy LEGEND Refugee location NYONG ET SO’O Refugee Camp OCÉAN MVILA UNHCR Representation DJA ET LOBO BOUMBA Bela SUD ET NGOKO Libongo UNHCR Sub-Office VALLÉE DU NTEM UNHCR Field Office UNHCR Field Unit Region boundary Departement boundary Roads GABON EQUATORIAL 100 Km CONGO ± GUINEA The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations Sources: Esri, USGS, NOAA Source: IOM, OCHA, UNHCR – Novembre 2019 Pour plus d’information, veuillez contacter Jean Luc KRAMO ([email protected]). -

Mountain Resources Expliotation For
International Journal of Geography and Régional Planning Research Vol.1,No.1,pp.1-12, March 2014 Published by European Centre for Research Training and Development UK (www.eajournals.org) MONTANE RESOURCES EXPLOITATION AND THE EMERGENCE OF GENDER ISSUES IN SANTA ECONOMY OF THE WESTERN BAMBOUTOS HIGHLANDS, CAMEROON Zephania Nji FOGWE Department of Geography, Box 3132, F.L.S.S., University of Douala ABSTRACT: Highlands have played key roles in the survival history of humankind. They are refuge heavens of valuable resources like fresh water endemic floral and faunal sanctuaries and other ecological imprints. The mountain resource base in most tropical Africa has been mined rather than managed for the benefit of the low-lying areas. The world over an appreciable population derives its sustenance directly from mountain resources and this makes for about one-tenth of the world’s poorest.The Western highlands of Cameroon are an archetypical territory of a high population density and an economically very active population. The highlands are characterised by an ecological fragility and a multi-faceted socio-economic dynamism at varied levels of poverty, malnutrition and under employment, yet about 80 percent of the Santa highlands’ population depends on its natural resource base of vast fertile land, fresh water and montane refuge forest for their livelihood. KEYWORDS: Montane Resources, Gender Issues, Santa Economy, Western Bamboutos, Cameroon INTRODUCTION The volcanic landscape on the western slopes of the Bamboutos mountain range slopes to the Santa Highlands is an area where agriculture in the form of crop production and animal rearing thrives with remarkable success. Arabica coffee cultivation was in extensive hectares cultivated at altitudes of about 1700m at the Santa Coffee Estate at Mile 12 in the 1970s and 1980s. -

Landscape Evolution, Neotectonics and Quaternary Environmental Change in Southern Cameroon
PALAEOECOLOGY OF AFRICA PALAEOECOLOGY OF AFRICA International Yearbook of Landscape Evolution and Palaeoenvironments 31 31 International Yearbook of Landscape Evolution and Palaeoenvironments 31 Runge Founded in 1966, the internationally recognized and acclaimed Series ‘Palaeoecology of Africa’ publishes interdisciplinary scientific papers on landscape evolution and on former environments of the African continent. Beginning with topics such as changes in climate and vegetation cover, the papers expand horizons and interconnections to various types of environmental dynamics from the Cainozoic up to the present; moreover, the aspect of human influence since the Late Quaternary is related to many of the areas studied. Cameroon Southern in Change Environmental Quaternary and Neotectonics Evolution, Landscape Volume 31 presents four comprehensive papers on long‐ and short‐term processes of landscape evolution (geological history, neotectonics and proxy Quaternary alluvia), as well as a recent regional perspective on environmental problems in Southern Cameroon. The book acts as a showcase for successful North‐South cooperation and capacity building for empowering African Universities. It is problem oriented and applied, and illustrates how scientific and interdisciplinary cooperation can work. In the framework of the German Research Foundation’s (DFG, Deutsche Forschungsgemeinschaft) funded "Rain Forest Savanna Contact" project (2003‐2009) two abbreviated English versions of PhD theses are here published, one by J. Eisenberg on neotectonics and the other by M. Sangen on river sediments in rain forest‐savanna transitional zones. Complementary articles are an introduction on geological history, by B. Kankeu et al. and a paper on environmental risks by M. Tchindjang et al., together these complete the results of this joint German‐Cameroonian research project. -

Full Text Article
SJIF Impact Factor: 3.458 WORLD JOURNAL OF ADVANCE ISSN: 2457-0400 Alvine et al. PageVolume: 1 of 3.21 HEALTHCARE RESEARCH Issue: 4. Page N. 07-21 Year: 2019 Original Article www.wjahr.com ASSESSING THE QUALITY OF LIFE IN TOOTHLESS ADULTS IN NDÉ DIVISION (WEST-CAMEROON) Alvine Tchabong1, Anselme Michel Yawat Djogang2,3*, Michael Ashu Agbor1, Serge Honoré Tchoukoua1,2,3, Jean-Paul Sekele Isouradi-Bourley4 and Hubert Ntumba Mulumba4 1School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes; Bangangté, Cameroon. 2School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes; Bangangté, Cameroon. 3Laboratory of Microbiology, Université des Montagnes Teaching Hospital; Bangangté, Cameroon. 4Service of Prosthodontics and Orthodontics, Department of Dental Medicine, University of Kinshasa, Kinshasa, Democratic Republic of Congo. Received date: 29 April 2019 Revised date: 19 May 2019 Accepted date: 09 June 2019 *Corresponding author: Anselme Michel Yawat Djogang School of Pharmacy, Higher Institute of Health Sciences, Université des Montagnes; Bangangté, Cameroon ABSTRACT Oral health is essential for the general condition and quality of life. Loss of oral function may be due to tooth loss, which can affect the quality of life of an individual. The aim of our study was to evaluate the quality of life in toothless adults in Ndé division. A total of 1054 edentulous subjects (partial, mixed, total) completed the OHIP-14 questionnaire, used for assessing the quality of life in edentulous patients. Males (63%), were more dominant and the ages of the patients ranged between 18 to 120 years old. Caries (71.6%), were the leading cause of tooth loss followed by poor oral hygiene (63.15%) and the consequence being the loss of aesthetics at 56.6%. -

Options for a National Culture Symbol of Cameroon: Can the Bamenda Grassfields Traditional Dress Fit?
EAS Journal of Humanities and Cultural Studies Abbreviated Key Title: EAS J Humanit Cult Stud ISSN: 2663-0958 (Print) & ISSN: 2663-6743 (Online) Published By East African Scholars Publisher, Kenya Volume-2 | Issue-1| Jan-Feb-2020 | DOI: 10.36349/easjhcs.2020.v02i01.003 Research Article Options for a National Culture Symbol of Cameroon: Can the Bamenda Grassfields Traditional Dress Fit? Venantius Kum NGWOH Ph.D* Department of History Faculty of Arts University of Buea, Cameroon Abstract: The national symbols of Cameroon like flag, anthem, coat of arms and seal do not Article History in any way reveal her cultural background because of the political inclination of these signs. Received: 14.01.2020 In global sporting events and gatherings like World Cup and international conferences Accepted: 28.12.2020 respectively, participants who appear in traditional costume usually easily reveal their Published: 17.02.2020 nationalities. The Ghanaian Kente, Kenyan Kitenge, Nigerian Yoruba outfit, Moroccan Journal homepage: Djellaba or Indian Dhoti serve as national cultural insignia of their respective countries. The https://www.easpublisher.com/easjhcs reason why Cameroon is referred in tourist circles as a cultural mosaic is that she harbours numerous strands of culture including indigenous, Gaullist or Francophone and Anglo- Quick Response Code Saxon or Anglophone. Although aspects of indigenous culture, which have been grouped into four spheres, namely Fang-Beti, Grassfields, Sawa and Sudano-Sahelian, are dotted all over the country in multiple ways, Cameroon cannot still boast of a national culture emblem. The purpose of this article is to define the major components of a Cameroonian national culture and further identify which of them can be used as an acceptable domestic cultural device. -

Dictionnaire Des Villages Du Mbam P
OFFICE DE LA RECHERCHE REPUBliQUE FEDERALE SCIENTIFIQUE ET TECHNIQUE DU OUT,RE·MER CAMEROUN CENTRE OR5TOM DE YAOUNDE DICTIONNAIRE DES _VILLAGES DU MBAM D'après la documentation réunie par ~la Section de Géographie de l'I.R.CAM.3 REPERTQIRE GEOGRAPHIQUE DU CAMEROUN FASCICULE n° 1 1 rR-GAM 8. P. '9J SH. n° 31 YAOUNDÉ Mai 1966 REPERTOIRE GEOGRAPHIQUE DU CAMEROUN Fasc. Tableau de la population du Cameroun, 68 p. Fév. 1965 SH, N° 17 Fasc. 2 Dictionnaire des villages du Dia et Lobo, 89 p. Juin 1965 SH. N° 22 Fasc. 3 Dictionnaire des villages de la Haute-Sanaga, 53 p. Août 1965 SH. N° 23 Fasc. 4 Dictionnaire des villages du Nyong et Mfoumou, 49 p. Octobre 1965 SH. N° 24 Fasc. 5 Dictionnaire des villages du Nyong et Soo 45 p. Novembre 1965 SH. N° 25 Fasc. 6 Dictionnaire des villages du Ntem 126 p. Décembre 1965 SH. N° 26 Fasc. 7 Dictionnaire- des villages de la Mefou 108 p. Janvier 1966 SH. N° 27 Fasc. 8 Dictionnaire des villages du Nyong et Kellé 51 p. Février 1966 SH. N° 28 Fasc. 9 Dictionnaire des villages de la Lékié 71 p. Mars 1966 SH. N° 29 Fasc. 10 Dictionnaire des villages de Kribi P. Mars 1966 SH. N° 30 Fasc. 11 Dictionnaire des villages du Mbam P. 60 Mai 1966 SH. N° 31 Fasc. 12 Dictionnaire des villages de Boumba Ngoko (en préparation) Fasc. 13 ùictionnaire des villages de Lom-et-Diérem (en préparation! omCE DE LA RECHERCHE SCIENTIFIQUE RERJBLlQUE FEDERALE ET TECHNI~E OUmE-MER ID CAMEROUN _ • _cee- -- - CENTRE ORSTOM DE YAOUNDE DICTIONNAIRE ~ VILLAGES DU ...........M B A M MAI 1!66 S.R. -

Ouest Uest E
quête d’assurance quête en transfrontaliers clients Les le Maire et son adjoint et son le Maire Kwemo Pierre fait enfermer ErnestOuandié honorent Patient etNdom Walla Kah Basile, Louka Adamou, Koupit Ouest Echos N° 1171 du 20 du 1171 N° Echos Ouest AFRILAND QUITTE GUINÉE LA EQUATO, LES PRODUCTEURS DE L’OUEST INQUIETS... Récipissé N°341 / RDDJ / C19 / BAPP DEVOIR DE MÉMOIRE : MÉMOIRE DE DEVOIR Since 1994 (vingt troisième année) O O COMMUNE DEBANWA COMMUNE PremierJournal national d’informations Régionales uest uest au 26 Janvier 2021 2021 Janvier 26 au l’arbitrage du Premier Ministre choix dusite et demandent Les populations s’opposent au première chaire Pierre Castel du pays L’Université deDschang hôte de la PARTENARIAT PRIVÉAU CAMEROUN PUBLIC PARTENARIAT CONSTRUCTION DU PEAGE AUTOMATIQUE DEBANDJA AUTOMATIQUE PEAGE DU CONSTRUCTION Tomaïno Ndam Njoya à Foumban à Njoya Ndam Tomaïno Awa se Fonka brûle au contact de E E INSTALLATION DU PRÉFET DU PRÉFET DU NOUN INSTALLATION Michel Eclador PEKOUA Eclador Michel publication de Directeur Les Monts d’Or 2020 2020 d’Or Monts Les Prix : 400 F. CFA F. 400 : Prix LA RÉGION DEL’OUEST RÉGION LA CÉLÈBRE SES AWARDS SES CÉLÈBRE c’est bientôt !!! bientôt c’est Actualités INSTALLATION DU NOUVEAU PRÉFET DU NOUN : Le gouverneur Awa Fonka perd sa sérénité à Foumban La scène a médusé les milliers de populations venus accueillir à la place des fêtes de Foumban, le nouveau préfet du département du Noun, Um Donacien, nommé le 18 décembre 2020 en remplacement de monsieur Boyomo Donatien, muté. l'occasion de la cérémonie de gramme de la plus leur temps à afficher leur mul- Njoya. -

Dictionnaire Des Villages De La Haute Sanaga a Été Entièrement Remise À Jour Et Corrigée En Fonction Des Derniers Renseignements Que Nous Possédons
OFFICE DE LA RECHERCHE REPUBLIQUE FEDEIlA~ SCIENTIFIQUE ET TECHNIQUE DU OUTRE-MER CAMEROUN CENTRE ORSTOM DE YAOUNDE DICTIONNAIR'E DES VILLAGES DE LA HAUTE SANAGA (2ème Editan) ~aprèS la documentation réunie ~-:-l ~ection de Géographie de l' ORST~ "~fUTOJkGEOGRAPHIQUE DU CAMEROUN FASCICULE N° 3 B. P. 193 SH. n° 50 YAOUNDE Août 1968 REPERTOIRE GEOGRAPHIQUE DU CAMEROUN Fasc. Tableau de la population du Cameroun, 68 p. Fév. 1965 SH. N° 17 Fasc. 2 Dictionnaire des villages du Dia et lobo, 89 p. Juin 1965 SH. N° 22 Fasc. 3 Dictionnaire des villages de la Haute-Sanaga, 44 p. Août 1968 SH. N° 50 (2éme édition) Fasc. 4 Dictionnaire des villages du Nyong et Mfoumou, 49 p. Octobre 1965 SH. N° 24 ~: Fasc. 5 Dictio~riaire des villages du Nyong et Soo 45 p. Novembre 1965 SH. N° 25 Fasc. 6 Dictionnaire des villages du Ntem 102 p. Juin 1968 SH. N° 46 (2ème édition) Fasc. 7 Dictionnaire des villages de la Mefou 108 p. Janvier 1966 SH. N° 27 Fa sc. 8 Dictionnaire des villages du Nyong et Kellé 51 p. Février 1966 SH. N° 28 Fa sc. 9 Dictionnaire des villages de la lékié 71 p. Mars 1966 SH. N° 29 Fasc. 10 Dictionnaire des villages de Kribi P. Mars 1966 SH. N° 30 Fasc. 11 Dictionnaire des villages du Mbam 60 P. Mai 1966 SH. N° 31 Fasc. 12 Dictionnaire des villages de Boumba Ngoko 34 p. Juin 1966 SH. 39 Fasc. 13 Dictionnaire des villages de lom-et-Djérem 35 p. Juillet 1967 SH. 40 Fasc. 14 Dictionnaire des villages de la Kadei 52 p. -

Assessing Cost Efficiency Among Small Scale Rice Producers in the West Region of Cameroon: a Stochastic Frontier Model Approach
International Journal of Humanities Social Sciences and Education (IJHSSE) Volume 3, Issue 6, June 2016, PP 1-7 ISSN 2349-0373 (Print) & ISSN 2349-0381 (Online) http://dx.doi.org/10.20431/2349-0381.0306001 www.arcjournals.org Assessing Cost Efficiency among Small Scale Rice Producers in the West Region of Cameroon: A Stochastic Frontier Model Approach Djomo, Raoul Fani*1, Ewung, Bethel1, Egbeadumah, Maryanne Odufa2 1Department of Agricultural Economics. University of Agriculture, Makurdi. Benue-State, Nigeria PMB 2373, Makurdi 2Department of Agricultural Economics and Extension, Federal University Wukari, PMB 1020 Wukari. Taraba State, Nigeria *[email protected] Abstract: Local farmers’ techniques and knowledge in rice cultivation being deficient and the aid system and research to support such farmers’ activities likewise insufficient, technological progress and increase in rice production are not yet assured. Therefore, this Study was undertaken to assess cost efficiency among small scale rice farmers in the West Region of Cameroon using stochastic frontier model approach. A purposive, multistage and stratified random sampling technique was used in selecting the respondents. A total of 192 small scale rice farmers were purposively selected from four (4) out of eight divisions. Data were collected using structured questionnaires and interview schedule, administered on the respondents were analyzed using descriptive statistics and stochastic frontier cost functions. The result indicates that the coefficient of cost of labour was found positive and significantly influence cost of production in small scale rice production at 1 percent level of probability, implying that increases in cost of labour by one unit will also increase cost of production in small scale rice farming by the value of it coefficient. -

N I G E R I a C H a D Central African Republic Congo
CAMEROON: LOCATIONS OF UNHCR PERSONS OF CONCERN (September 2020) ! PERSONNES RELEVANT DE Maïné-Soroa !Magaria LA COMPETENCE DU HCR (POCs) Geidam 1,951,731 Gashua ! ! CAR REFUGEES ING CurAi MEROON 306,113 ! LOGONE NIG REFUGEES IN CAMEROON ET CHARI !Hadejia 116,409 Jakusko ! U R B A N R E F U G E E S (CENTRAL AFRICAN REPUBLIC AND 27,173 NIGERIAN REFUGEE LIVING IN URBAN AREA ARE INCLUDED) Kousseri N'Djamena !Kano ASYLUM SEEKERS 9,332 Damaturu Maiduguri Potiskum 1,032,942 INTERNALLY DISPLACED PERSO! NS (IDPs) * RETURNEES * Waza 484,036 Waza Limani Magdeme Number of refugees MAYO SAVA Mora ! < 10,000 EXTRÊME-NORD Mokolo DIAMARÉ Biu < 50,000 ! Maroua ! Minawao MAYO Bauchi TSANAGA Yagoua ! Gom! be Mubi ! MAYO KANI !Deba MAYO DANAY < 75000 Kaele MAYO LOUTI !Jos Guider Number! of IDPs N I G E R I A Lafia !Ləre ! < 10,000 ! Yola < 50,000 ! BÉNOUÉ C H A D Jalingo > 75000 ! NORD Moundou Number of returnees ! !Lafia Poli Tchollire < 10,000 ! FARO MAYO REY < 50,000 Wukari ! ! Touboro !Makurdi Beke Chantier > 75000 FARO ET DÉO Tingere ! Beka Paoua Number of asylum seekers Ndip VINA < 10,000 Bocaranga ! ! Borgop Djohong Banyo ADAMAOUA Kounde NORD-OUEST Nkambe Ngam MENCHUM DJEREM Meiganga DONGA MANTUNG MAYO BANYO Tibati Gbatoua Wum BOYO MBÉRÉ Alhamdou !Bozoum Fundong Kumbo BUI CENTRAL Mbengwi MEZAM Ndop MOMO AFRICAN NGO Bamenda KETUNJIA OUEST MANYU Foumban REPUBLBICaoro BAMBOUTOS ! LEBIALEM Gado Mbouda NOUN Yoko Mamfe Dschang MIFI Bandjoun MBAM ET KIM LOM ET DJEREM Baham MENOUA KOUNG KHI KOUPÉ Bafang MANENGOUBA Bangangte Bangem HAUT NKAM Calabar NDÉ SUD-OUEST -

Cameroon 2014 Table A
Cameroon 2014 Table A: Total funding and outstanding pledges* as of 29 September 2021 http://fts.unocha.org (Table ref: R10) Compiled by OCHA on the basis of information provided by donors and appealing organizations. Donor Channel Description Funding Outstanding Pledges USD USD Allocation of unearmarked funds by FAO The assistance is intended to cover the immediate needs of 493,000 0 FAO refugees and host populations on agricultural inputs to enable households engaged in agricultural activities boost vegetable production during the off-season 2014 (August to November). This should improve food security and generate income through the sale of surpluses. In addition, in order to promote a more sustainable approach, the project will provide cereals and cassava processing facilities in host villages, coupled with training sessions. This will reduce post-harvest losses in corn and cassava, and thus help to strengthen the resilience of target populations. Bill and Melinda Gates Foundation IMC to respond to the cholera outbreak in the Far North region in 900,000 0 Cameroon and to address the humanitarian needs of CAR refugees in the East and Adamawa regions of Cameroon Canada IFRC IFRC DREF operation MDRCM015 in Cameroon - IFRC 32,802 0 December 2013 operation to assist CAR refugees in Cameroon (M013807) Carry-over (donors not specified) WFP to be allocated to specific project 31,201 0 Central Emergency Response Fund WHO Medical assistance to local communities and refugees in 55,774 0 response to ongoing nutritional crisis, cholera and measles -

Evaluating the Constraints and Opportunities of Maize Production in the West Region of Cameroon for Sustainable Development
Journal of Sustainable Development in Africa (Volume 13, No.4, 2011) ISSN: 1520-5509 Clarion University of Pennsylvania, Clarion, Pennsylvania EVALUATING THE CONSTRAINTS AND OPPORTUNITIES OF MAIZE PRODUCTION IN THE WEST REGION OF CAMEROON FOR SUSTAINABLE DEVELOPMENT Godwin Anjeinu Abu1, Raoul Fani Djomo-Choumbou1 and Stephen Adogwu Okpachu2 1. Department of Agricultural Economics, University of Agriculture PMB 2373, Makurdi Benue State- Nigeria 2. Federal College of Education (Technical), Potiskum, Yobe State, Nigeria ABSTRACT A study of Evaluating the constraints and opportunities of Maize production in the West Region of Cameroon was carried out using primary and secondary data collected. One hundred and twenty (120) maize farmers randomly selected from eight (8) villages were interviewed using structured questionnaire. Data from the study were analyzed using descriptive statistics such as frequency distribution, percentages, and inferential statistics such as multiple linear regressions. The study found that most maize farmers in the study area were small scale farmers and are full time farmers, the major maize production constraints was poor access to credit facilities. it was also found any unit increase in the quantity of any of the resources used for maize production will increase maize output by the value of their estimated coefficients respectively , however to raise maize production, the study recommend that financial institutions such as agricultural and community banks should be established in the study area with the simple procedure of securing loans. The relevant government agencies and non- governmental organization should mobilize the maize farmers to form themselves into formidable group so that they can derive maximum benefit of collective union.