Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
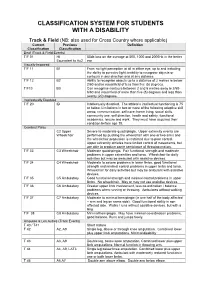
Disability Classification System
CLASSIFICATION SYSTEM FOR STUDENTS WITH A DISABILITY Track & Field (NB: also used for Cross Country where applicable) Current Previous Definition Classification Classification Deaf (Track & Field Events) T/F 01 HI 55db loss on the average at 500, 1000 and 2000Hz in the better Equivalent to Au2 ear Visually Impaired T/F 11 B1 From no light perception at all in either eye, up to and including the ability to perceive light; inability to recognise objects or contours in any direction and at any distance. T/F 12 B2 Ability to recognise objects up to a distance of 2 metres ie below 2/60 and/or visual field of less than five (5) degrees. T/F13 B3 Can recognise contours between 2 and 6 metres away ie 2/60- 6/60 and visual field of more than five (5) degrees and less than twenty (20) degrees. Intellectually Disabled T/F 20 ID Intellectually disabled. The athlete’s intellectual functioning is 75 or below. Limitations in two or more of the following adaptive skill areas; communication, self-care; home living, social skills, community use, self direction, health and safety, functional academics, leisure and work. They must have acquired their condition before age 18. Cerebral Palsy C2 Upper Severe to moderate quadriplegia. Upper extremity events are Wheelchair performed by pushing the wheelchair with one or two arms and the wheelchair propulsion is restricted due to poor control. Upper extremity athletes have limited control of movements, but are able to produce some semblance of throwing motion. T/F 33 C3 Wheelchair Moderate quadriplegia. Fair functional strength and moderate problems in upper extremities and torso. -

Reframing Sport Contexts: Labeling, Identities, and Social Justice
Reframing Sport Contexts: Labeling, Identities, and Social Justice Dr. Ted Fay and Eli Wolff Sport in Society Disability in Sport Initiative Northeastern University Critical Context • Marginalization (Current Status Quo) vs. • Legitimatization (New Inclusive Paradigm) Critical Context Naturalism vs. Trans-Humanism (Wolbring, G. (2009) How Do We Handle Our Differences related to Labeling Language and Cultural Identities? • Stereotyping? • Prejudice? • Discrimination? (Carr-Ruffino, 2003, p. 1) Ten Major Cultural Differences 1) Source of Control 2) Collectivism or Individualism 3) Homogeneous or Heterogeneous 4) Feminine or Masculine 5) Rank Status 6) Risk orientation 7) Time use 8) Space use 9) Communication Style 10) Economic System (Carr – Ruffino, 2003, p.27) Rationale for Inclusion • Divisioning by classification relative to “fair play” and equity principles • Sport model rather than “ism” segregated model (e.g., by race, gender, disability, socio-economic class, sexual orientation, look (body image), sect (religion), age) • Legitimacy • Human rights and equality Social Dynamics of Inequality Reinforce and reproduce Social Institutions Ideology Political (Patriarchy) Economic Educational Perpetuates Religious Prejudice & Are institutionalized by Discrimination Cultural Practices (ISM) Sport Music Art (Sage, 1998) Five Interlinking Conceptual Frameworks • Critical Change Factors Model (CCFM) • Organizational Continuum in Sport Governance (OCSG) • Criteria for Inclusion in Sport Organizations (CISO) • Individual Multiple Identity Sport Classifications Index (IMISCI) • Sport Opportunity Spectrum (SOS) Critical Change Factors Model (CCFM) F1) Change/occurrence of major societal event (s) affecting public opinion toward ID group. F2) Change in laws, government and court action in changing public policies toward ID group. F3) Change in level of influence of high profile ID group role models on public opinion. -

Connexion Client Cataloging Quick Reference
OCLC Connexion Client Cataloging Quick Reference Introduction Keystroke shortcuts The Connexion client is a Windows-based interface to OCLC • Use default keystroke shortcuts or assign your own to activate Connexion® used to access WorldCat for cataloging. commands, insert characters, run macros, and insert text strings. This quick reference provides brief instructions for editing, saving, • View key assignments in View > Assigned Keys.To print or copy exporting, and printing labels for bibliographic records; using local files; the list, click Print or Copy to Clipboard. creating and adding records to WorldCat; replacing WorldCat records; Tip: Before printing, click a column heading to sort the list by data batch processing; and cataloging with non-Latin scripts. in the column. • Assign your own keystrokes in Tools > Keymaps. Multiscript support: The client supports the following non-Latin scripts: • Print a function key template to put at the top of your keyboard: Arabic, Armenian, Bengali, Chinese, Cyrillic, Devanagari, Ethiopic, Greek, Hebrew, Japanese, Korean, Syriac, Tamil, and Thai. www.oclc.org/support/documentation/connexion/client/ gettingstarted/keyboardtemplate.pdf. This quick reference does not cover instructions for authorities work or instructions already available in: Toolbar • Getting Started with Connexion Client • The client installs with three toolbars displayed by default: • Connexion Client Setup Worksheet o Main client toolbar (with command-equivalent buttons) • Connexion: Searching WorldCat Quick Reference o WorldCat quick search tool Quick tools for text strings and user tools Connexion client documentation assumes knowledge of MARC o cataloging. • Customize the main client toolbar: In Tools > Toolbar Editor, drag and drop buttons to add or remove, or reset to the default. -

Spinal Fractures Classification System an Aospine Knowledge Forum Initiative
Spinal Fractures Classification System an AOSpine Knowledge Forum initiative Subaxial Spine Fractures Thoracolumbar Spine Fractures Sacral Spine Fractures AOSpine–the leading global academic community for innovative education and research in spine care, inspiring lifelong learning and improving patients’ lives. Spinal Fractures Classification System 2 Spinal Fractures Classification System an AOSpine Knowledge Forum initiative CONTENT AOSpine Classification and Injury Severity System ................ 04 for Traumatic Fractures of the Subaxial Spine AOSpine Classification and Injury Severity System ................. 37 for Traumatic Fractures of the Thoracolumbar Spine AOSpine Classification and Injury Severity System .................55 for Traumatic Fractures of the Sacral Spine Spinal Fractures Classification System 3 AOSpine Knowledge Forum AOSpine Classification and Injury Severity System for Traumatic Fractures of the Subaxial Spine This is the present form of the classification the AOSpine Knowledge Forum (KF) SCI & Trauma is working on. It is the aim of the KF to develop a system, which can in the future be used as a tool for scientific research and a guide for treatment. This system is being subjected to a rigorous scientific assessment. Project members Aarabi B, Bellabarba C, Chapman J, Dvorak M, Fehlings M, Kandziora F, Kepler C, (in alphabetic order) Oner C, Rajasekaran S, Reinhold M, Schnake K, Vialle L and Vaccaro A. Disclaimer 1. Vaccaro, A. R., J. D. Koerner, K. E. Radcliff, F. C. Oner, M. Reinhold, K. J. Schnake, F. Kandziora, M. G. Fehlings, M. F. Dvorak, B. Aarabi, S. Rajasekaran, G. D. Schroeder, C. K. Kepler and L. R. Vialle (2015). “AOSpine subaxial cervical spine injury classification system.” Eur Spine J. 2. International validation process to be completed in 2015. -
Modifier Chart
Modifier Chart 22 Increased procedural services 23 Unusual anesthesia Unrelated Evaluation & Management (E/M) services by the same 24 provider during postoperative period Significant, separately identifiable E/M service by the same physician 25 on the same day of the procedure or other service 26 Professional component 32 Mandated Services When primary purpose is the delivery of an evidence-based service in accord with US Preventive Services Task Force A or B rating in effect 33 and other preventive services identified in preventive services mandates (legislative or regulatory) 47 Anesthesia by surgeon 50 Bilateral procedure 51 Multiple procedures 52 Reduced services 53 Discontinued procedure 54 Surgical care only 55 Postoperative management only 57 Decision for surgery Staged or related procedure or service by the same physician during 58 the postoperative period 59 Distinct procedural service Separate encounter, distinct because it occurred during a separate XE * encounter Separate structure, distinct because it was performed on a separate XS * organ/structure Separate practitioner, distinct because it was performed by a XP * different practitioner Unusual non-overlapping service, distinct because it does not XL * overlap usual components of the main service 62 Two surgeons 63 Procedure performed on infants less than 4 kgs. 66 Surgical team Repeat procedure or service by the same physician or other qualified 76 health care professional Repeat procedure or service by another physician or other qualified 77 health care professional Unplanned -

Research Article KINEMATIC ANALYSIS of JAVELIN THROW
©Journal of Sports Science and Medicine (2003) 2, 36-46 http://www.jssm.org Research article KINEMATIC ANALYSIS OF JAVELIN THROW PERFORMED BY WHEELCHAIR ATHLETES OF DIFFERENT FUNCTIONAL CLASSES John W. Chow 1 *, Ann F. Kuenster 2 and Young-tae Lim 3 1Department of Exercise and Sport Sciences, University of Florida, Gainesville, USA 2Department of Kinesiology, University of Illinois at Urbana-Champaign, USA 3Division of Sport Science, Konkuk University, Chungju, Korea Received: 10 December 2002 / Accepted: 07 February 2003 / Published (online): 01 June 2003 ABSTRACT The purpose of this study was to identify those kinematic characteristics that are most closely related to the functional classification of a wheelchair athlete and measured distance of a javelin throw. Two S- VHS camcorders (60 field· s-1) were used to record the performance of 15 males of different classes. Each subject performed 6 - 10 throws and the best two legal throws from each subject were selected for analysis. Three-dimensional kinematics of the javelin and upper body segments at the instant of release and during the throw (delivery) were determined. The selection of kinematic parameters that were analyzed in this study was based on a javelin throw model showing the factors that determine the measured distance of a throw. The average of two throws for each subject was used to compute Spearman rank correlation coefficients between selected parameters and measured distance, and between selected parameters and the functional classification. The speeds and angles of the javelin at release, ranged from 9.1 to 14.7 m· s-1 and 29.6 to 35.8º, respectively, were smaller than those exhibited by elite male able-bodied throwers. -

Letters to Assessors 2006-010
STATE OF CALIFORNIA BETTY T. YEE STATE BOARD OF EQUALIZATION Acting Member PROPERTY AND SPECIAL TAXES DEPARTMENT First District, San Francisco 450 N STREET, SACRAMENTO, CALIFORNIA PO BOX 942879, SACRAMENTO, CALIFORNIA 94279-0064 BILL LEONARD Second District, Sacramento/Ontario 916 445-4982 FAX 916 323-8765 www.boe.ca.gov CLAUDE PARRISH Third District, Long Beach February 6, 2006 JOHN CHIANG Fourth District, Los Angeles STEVE WESTLY State Controller, Sacramento RAMON J. HIRSIG Executive Director No. 2006/010 CORRECTION TO COUNTY ASSESSORS: REVENUE AND TAXATION CODE SECTION 69.5: PROPOSITIONS 60, 90, AND 110 Section 69.5 was added to the Revenue and Taxation Code1 in 1987 to implement Proposition 60, which amended section 2 of article XIII A of the California Constitution to authorize the Legislature to provide for the transfer of a base year value from a principal residence2 to a replacement dwelling within the same county by a homeowner age 55 and over. Subsequently, section 69.5 was amended to implement Proposition 90, which authorized county boards of supervisors to adopt ordinances allowing base year value transfers between different counties, and Proposition 110, which extended these provisions to severely and permanently disabled persons of any age. After summarizing the key elements of section 69.5, this letter provides answers to frequently asked questions about its application. This letter supersedes Letters To Assessors No. 87/71 (dated September 11, 1987) and No. 88/10 (dated February 11, 1988). SUMMARY OF SECTION 69.5 Section 69.5 allows a homeowner to transfer the existing base year value to a replacement dwelling provided that: • If the replacement property is located in a different county than the original property, then the county in which the replacement dwelling is located must have a current ordinance allowing base year value transfers from other counties. -

The Role of the First Five Formants in Three Vowels of Mandarin for Forensic Voice Analysis
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 The role of the first five formants in three vowels of mandarin for forensic voice analysis Cao, Honglin ; Dellwo, Volker Abstract: Formant characteristics are most commonly part of forensic speaker comparison (FSC). How- ever, only formants F1 to F3 typically occur in evidence material because it is mostly recorded via telephone. Given recent technological advances in telephony (e.g. WeChat or WhatsApp) higher for- mants (F4-F5) are becoming increasingly part of evidence material. The present study investigated the speaker- distinguishing properties of F1 to F5 of three sustained vowels /i/, /y/ and // in Mandarin produced by 20 young male speakers. Based on discriminant analysis, for each single formant, the best predictors were F5 for /i/ and F4 for /y/ and //. Classification performance varied between vowels. Inclusion of two and three formants yielded higher classification rates of 30−80%. The best value was provided by the combination of F2, F4 and F5 of //. The value and limitations of F4 and F5 for FSC are discussed. Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-177494 Conference or Workshop Item Published Version Originally published at: Cao, Honglin; Dellwo, Volker (2019). The role of the first five formants in three vowels of mandarin for forensic voice analysis. In: International Congress of Phonetic Sciences, Melbourne, 5 August 2019 - 9 August 2019, 617-621. THE ROLE OF THE FIRST FIVE FORMANTS IN THREE VOWELS OF MANDARIN FOR FORENSIC VOICE ANALYSIS Honglin Caoa,b,c &Volker Dellwoc a Key Laboratory of Evidence Science (China University of Political Science and Law), Ministry of Education, China. -

Women in the 2000, 2004 and 2008 Olympic and Paralympic Games an Analysis of Participation, Leadership and Media Opportunities
September 2009 Women in the 2000, 2004 and 2008 Olympic and Paralympic Games An Analysis of Participation, Leadership and Media Opportunities A Women’s Sports Foundation Research Report Authorship and Acknowledgments TThis report was authored by Maureen Smith, Ph.D., California State University, Sacramento, and Alison M. Wrynn, Ph.D., California State University, Long Beach. The report was reviewed by Donna A. Lopiano, Ph.D.; Don Sabo, Ph.D.; Marjorie A. Snyder, Ph.D.; Linda Mastandrea; Terri Lakowski; Carly Adams, Ph.D., University of Lethbridge; Ellen Carlton, Ph.D., Sonoma State University; Kerrie Kauer, Ph.D., California State University, Long Beach; Cheryl Cooky, Ph.D., California State University, Fullerton; Matthew Llewellyn, Pennsylvania State University; and Jennifer Piatt, Ph.D., California State University, Sacramento. The initial data collection was assisted by graduate students at California State University, Sacramento: Brandon Babcock, Kristi Jouett, Fred Kelley, Louis Lopez, Lindsey McEuen, Rusty Price, K.V. Vigil and Kelli White as well as graduate students from California State University, Long Beach. All data that was obtained from the Internet was accurate as of April 2009. Every attempt was made to obtain the most accurate and up-to-date data for this report. Special thanks to Deana Monahan for her editorial and graphic design expertise. Published September 2009, by the Women’s Sports Foundation® Eisenhower Park, 1899 Hempstead Turnpike, Suite 400 East Meadow, NY 11554 [email protected] www.WomensSportsFoundation.org © 2009, Women’s Sports Foundation, All Rights Reserved This report may be downloaded from www.WomensSportsFoundation.org. This report may be reproduced and distributed only in its entirety. -
Critical Evaluation of Deep Neural Networks for Wrist Fracture Detection
www.nature.com/scientificreports OPEN Critical evaluation of deep neural networks for wrist fracture detection Abu Mohammed Raisuddin1,5*, Elias Vaattovaara1,2,5, Mika Nevalainen1,2, Marko Nikki2, Elina Järvenpää2, Kaisa Makkonen2, Pekka Pinola1,2, Tuula Palsio1,4, Arttu Niemensivu1, Osmo Tervonen1,2 & Aleksei Tiulpin1,2,3 Wrist Fracture is the most common type of fracture with a high incidence rate. Conventional radiography (i.e. X-ray imaging) is used for wrist fracture detection routinely, but occasionally fracture delineation poses issues and an additional confrmation by computed tomography (CT) is needed for diagnosis. Recent advances in the feld of Deep Learning (DL), a subfeld of Artifcial Intelligence (AI), have shown that wrist fracture detection can be automated using Convolutional Neural Networks. However, previous studies did not pay close attention to the difcult cases which can only be confrmed via CT imaging. In this study, we have developed and analyzed a state-of-the-art DL-based pipeline for wrist (distal radius) fracture detection—DeepWrist, and evaluated it against one general population test set, and one challenging test set comprising only cases requiring confrmation by CT. Our results reveal that a typical state-of-the-art approach, such as DeepWrist, while having a near- perfect performance on the general independent test set, has a substantially lower performance on the challenging test set—average precision of 0.99 (0.99–0.99) versus 0.64 (0.46–0.83), respectively. Similarly, the area under the ROC curve was of 0.99 (0.98–0.99) versus 0.84 (0.72–0.93), respectively. -

A Key Method in Vietnam's Suppression of Covid-19
The ‘F-System’ of Targeted Isolation: A Key Method In Vietnam’s Suppression of Covid-19 Andrew Hardy, Melody Shum, Vũ Ngọc Quyên To cite this version: Andrew Hardy, Melody Shum, Vũ Ngọc Quyên. The ‘F-System’ of Targeted Isolation: A Key Method In Vietnam’s Suppression of Covid-19. 2020. halshs-03151062 HAL Id: halshs-03151062 https://halshs.archives-ouvertes.fr/halshs-03151062 Submitted on 2 Mar 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. EUROPEAN POLICY BRIEF THE ‘F-SYSTEM ’ OF TARGETED ISOLATION : A KEY METHOD IN VIETNAM ’S SUPPRESSION OF COVID -19 Andrew Hardy, Melody Shum, V ũ Ng ọc Quyên 1, December 2020 INTRODUCTION Figure 1. Vietnam Covid-19 statistics (3 August 2020). Data source: Vietnam Ministry of Health (MOH) Introduction: Targeted isolation Vietnam’s suppression of Covid-19 owes much to the use of a containment method based on targeted isolation. Targeting was done based on contact-tracing – isolating people exposed to infected persons – and on geography – isolating places where infections clustered. Both forms of targeting were relevant to the outcome, but here we examine the first, lesser-known form, the test, trace and isolate system for individuals, for which Vietnam developed an original approach. -

Wheeled Mobility and Seating Equipment Following Spinal Cord Injury
Wheeled Mobility and Seating Equipment Following Spinal Cord Injury Laura Titus PhD OT Lindsey Guilbault MScPT Ben Mortenson PhD Stephanie Cornell PT Susan Moir OT Jennifer Birt OT Brooke Benton BSc RDH The following people are greatfully acknowledged for their contribution to the growth of this chapter as past co-authors; Alba Casalino PT, Amanda McIntyre RN, Sandra Connolly OT, Sarah Miles OT, Karen Trenholm PT and, Mary Ann Regan RN. www.scireproject.com Version 7.0 Key Points The evidence suggests that stroke pattern use varies based on individual preference and the environmental demands with some stroke patterns being more effective to achieve specific outcomes. The evidence supports that to avoid accumulating shoulder impingement stresses proper propulsion technique must be considered based on a combination of kinematics (e.g., contact angle, stroke frequency, movement patterns at each joint), stroke pattern, wheelchair fit and set up. Neck, trunk, scapular, clavicle, elbow, wrist and shoulder kinetics and kinematics singly or cumulatively influence the efficacy of manual wheelchair propulsion and therefore all should be considered in propulsion efficiency as well as in propulsion- related injuries, particularly if propulsion speed or surface slope increases. The push and recovery phases of propulsion both need to be considered in relation to manual wheelchair propulsion as the kinetics and kinematics differ, and differ between people with paraplegia and tetraplegia, which therefore have implications for propulsion training in the clinical setting. The following need to be considered in relation to propulsion and back support height; a) effect on propulsion cadence; b) amount of shoulder range of motion used and; c) the length of the push stroke (i.e., length between the start and end position of the hand on the rim).