Critical Evaluation of Deep Neural Networks for Wrist Fracture Detection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Disability Classification System
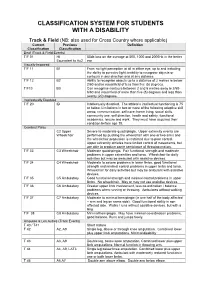
CLASSIFICATION SYSTEM FOR STUDENTS WITH A DISABILITY Track & Field (NB: also used for Cross Country where applicable) Current Previous Definition Classification Classification Deaf (Track & Field Events) T/F 01 HI 55db loss on the average at 500, 1000 and 2000Hz in the better Equivalent to Au2 ear Visually Impaired T/F 11 B1 From no light perception at all in either eye, up to and including the ability to perceive light; inability to recognise objects or contours in any direction and at any distance. T/F 12 B2 Ability to recognise objects up to a distance of 2 metres ie below 2/60 and/or visual field of less than five (5) degrees. T/F13 B3 Can recognise contours between 2 and 6 metres away ie 2/60- 6/60 and visual field of more than five (5) degrees and less than twenty (20) degrees. Intellectually Disabled T/F 20 ID Intellectually disabled. The athlete’s intellectual functioning is 75 or below. Limitations in two or more of the following adaptive skill areas; communication, self-care; home living, social skills, community use, self direction, health and safety, functional academics, leisure and work. They must have acquired their condition before age 18. Cerebral Palsy C2 Upper Severe to moderate quadriplegia. Upper extremity events are Wheelchair performed by pushing the wheelchair with one or two arms and the wheelchair propulsion is restricted due to poor control. Upper extremity athletes have limited control of movements, but are able to produce some semblance of throwing motion. T/F 33 C3 Wheelchair Moderate quadriplegia. Fair functional strength and moderate problems in upper extremities and torso. -

Reframing Sport Contexts: Labeling, Identities, and Social Justice
Reframing Sport Contexts: Labeling, Identities, and Social Justice Dr. Ted Fay and Eli Wolff Sport in Society Disability in Sport Initiative Northeastern University Critical Context • Marginalization (Current Status Quo) vs. • Legitimatization (New Inclusive Paradigm) Critical Context Naturalism vs. Trans-Humanism (Wolbring, G. (2009) How Do We Handle Our Differences related to Labeling Language and Cultural Identities? • Stereotyping? • Prejudice? • Discrimination? (Carr-Ruffino, 2003, p. 1) Ten Major Cultural Differences 1) Source of Control 2) Collectivism or Individualism 3) Homogeneous or Heterogeneous 4) Feminine or Masculine 5) Rank Status 6) Risk orientation 7) Time use 8) Space use 9) Communication Style 10) Economic System (Carr – Ruffino, 2003, p.27) Rationale for Inclusion • Divisioning by classification relative to “fair play” and equity principles • Sport model rather than “ism” segregated model (e.g., by race, gender, disability, socio-economic class, sexual orientation, look (body image), sect (religion), age) • Legitimacy • Human rights and equality Social Dynamics of Inequality Reinforce and reproduce Social Institutions Ideology Political (Patriarchy) Economic Educational Perpetuates Religious Prejudice & Are institutionalized by Discrimination Cultural Practices (ISM) Sport Music Art (Sage, 1998) Five Interlinking Conceptual Frameworks • Critical Change Factors Model (CCFM) • Organizational Continuum in Sport Governance (OCSG) • Criteria for Inclusion in Sport Organizations (CISO) • Individual Multiple Identity Sport Classifications Index (IMISCI) • Sport Opportunity Spectrum (SOS) Critical Change Factors Model (CCFM) F1) Change/occurrence of major societal event (s) affecting public opinion toward ID group. F2) Change in laws, government and court action in changing public policies toward ID group. F3) Change in level of influence of high profile ID group role models on public opinion. -

Pseudogout at the Knee Joint Will Frequently Occur After Hip Fracture
Harato and Yoshida Journal of Orthopaedic Surgery and Research (2015) 10:4 DOI 10.1186/s13018-014-0145-9 RESEARCH ARTICLE Open Access Pseudogout at the knee joint will frequently occur after hip fracture and lead to the knee pain in the early postoperative period Kengo Harato1,3*† and Hiroki Yoshida2† Abstract Background: Symptomatic knee joint effusion is frequently observed after hip fracture, which may lead to postoperative knee pain during rehabilitation after hip fracture surgery. However, unfortunately, very little has been reported on this phenomenon in the literature. The purpose of the current study was to investigate the relationship between symptomatic knee effusion and postoperative knee pain and to clarify the reason of the effusion accompanied by hip fracture. Methods: A total of 100 patients over 65 years of age with an acute hip fracture after fall were prospectively followed up. Knee effusion was assessed on admission and at the operating room before the surgery. If knee effusion was observed at thetimeofthesurgery,synovialfluidwascollectedintosyringes to investigate the cause of the effusion using a compensated polarized light microscope. Furthermore, for each patient, we evaluated age, sex, radiographic knee osteoarthritis (OA), type of the fracture, laterality, severity of the fracture, and postoperative knee pain during rehabilitation. These factors were compared between patients with and without knee effusion at the time of the surgery. As a statistical analysis, we used Mann–Whitney U-test for patients’ age and categorical variables were analyzed by chi-square test or Fisher’sexacttest. Results: A total of 30 patients presented symptomatic knee effusion at the time of the surgery. -

Connexion Client Cataloging Quick Reference
OCLC Connexion Client Cataloging Quick Reference Introduction Keystroke shortcuts The Connexion client is a Windows-based interface to OCLC • Use default keystroke shortcuts or assign your own to activate Connexion® used to access WorldCat for cataloging. commands, insert characters, run macros, and insert text strings. This quick reference provides brief instructions for editing, saving, • View key assignments in View > Assigned Keys.To print or copy exporting, and printing labels for bibliographic records; using local files; the list, click Print or Copy to Clipboard. creating and adding records to WorldCat; replacing WorldCat records; Tip: Before printing, click a column heading to sort the list by data batch processing; and cataloging with non-Latin scripts. in the column. • Assign your own keystrokes in Tools > Keymaps. Multiscript support: The client supports the following non-Latin scripts: • Print a function key template to put at the top of your keyboard: Arabic, Armenian, Bengali, Chinese, Cyrillic, Devanagari, Ethiopic, Greek, Hebrew, Japanese, Korean, Syriac, Tamil, and Thai. www.oclc.org/support/documentation/connexion/client/ gettingstarted/keyboardtemplate.pdf. This quick reference does not cover instructions for authorities work or instructions already available in: Toolbar • Getting Started with Connexion Client • The client installs with three toolbars displayed by default: • Connexion Client Setup Worksheet o Main client toolbar (with command-equivalent buttons) • Connexion: Searching WorldCat Quick Reference o WorldCat quick search tool Quick tools for text strings and user tools Connexion client documentation assumes knowledge of MARC o cataloging. • Customize the main client toolbar: In Tools > Toolbar Editor, drag and drop buttons to add or remove, or reset to the default. -

Treatment of Common Hip Fractures: Evidence Report/Technology
This report is based on research conducted by the Minnesota Evidence-based Practice Center (EPC) under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. HHSA 290 2007 10064 1). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help clinicians, employers, policymakers, and others make informed decisions about the provision of health care services. This report is intended as a reference and not as a substitute for clinical judgment. This report may be used, in whole or in part, as the basis for the development of clinical practice guidelines and other quality enhancement tools, or as a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. Evidence Report/Technology Assessment Number 184 Treatment of Common Hip Fractures Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA 290 2007 10064 1 Prepared by: Minnesota Evidence-based Practice Center, Minneapolis, Minnesota Investigators Mary Butler, Ph.D., M.B.A. Mary Forte, D.C. Robert L. Kane, M.D. Siddharth Joglekar, M.D. Susan J. Duval, Ph.D. Marc Swiontkowski, M.D. -

Fracture Lower Extremity Part II
CONTENTS FEMUR SHAFT BOTH BONE SUBTROCHANTERIC TIBIAL PLAFON FRACTURE LOWER FRACTURE ANKLE EXTREMITIES: PART 2 FRACTURE FEMUR FOOT SUPRACONDYLAR FRACTURE FEMUR CALCANEUS PATELLA TALUS WORAWAT LIMTHONGKUL, M.D. 14 JAN 2013 TIBIA LISFRANC’S TIBIAL PLATEAU METATARSAL 1 2 SUBTROCHANTERIC FRACTURE FEMUR A PART OF FRACTURE OCCUR BETWEEN TIP OF LESSER TROCHANTER AND A POINT 5 SUBTROCHANTERIC CM DISTALLY CALCAR FEMORALE FRACTURE LARGE FORCES ARE NEEDED TO CAUSE FRACTURES IN 5 CM YOUNG & ADULT INJURY IS RELATIVELY TRIVIAL IN ELDERLY 2° CAUSE: OSTEOPOROSIS, OSTEOMALACIA, PAGET’S 3 4 SUBTROCHANTERIC FRACTURE FEMUR TREATMENT INITIAL FEMUR SHAFT TRACTION DEFINITE FRACTURE ORIF WITH INTRAMEDULLARY NAIL OR 95 DEGREE HIP- SCREW-PLATE 5 6 FEMUR FRACTURE FILM HIPS SEVERE PAIN, UNABLE TO BEAR WEIGHT 10% ASSOCIATE FEMORAL SUPRACONDYLAR NECK FRACTURE FEMUR FRACTURE TREATMENT: ORIF WITH IM NAIL OR P&S COMPLICATION: HEMORRHAGE, NEUROVASCULAR INJURY, FAT EMBOLI 7 8 SUPRACONDYLAR FEMUR FRACTURE SUPRACONDYLAR ZONE DIRECT VIOLENCE IS THE USUAL CAUSE PATELLA FRACTURE LOOK FOR INTRA- ARTICULAR INVOLVEMENT CHECK TIBIAL PULSE TREATMENT: ORIF WITH P&S 9 10 PATELLA FRACTURE PATELLA FRACTURE FUNCTION: LENGTHENING THE ANTERIOR LEVER ARM DDX: BIPATITE PATELLA AND INCREASING THE (SUPEROLATERAL) EFFICIENCY OF THE QUADRICEPS. TREATMENT: DIRECT VS INDIRECT NON-DISPLACE, INJURY INTACT EXTENSOR : CYLINDRICAL CAST TEST EXTENSOR MECHANISM DISPLACE, DISRUPT EXTENSOR: ORIF WITH VERTICAL FRACTURE: TBW MERCHANT VIEW 11 12 PATELLAR DISLOCATION ADOLESCENT FEMALE DISLOCATION AROUND USUALLY -

Medical Consultation for the Elderly Patient with Hip Fracture
J Am Board Fam Pract: first published as 10.3122/15572625-11-5-366 on 1 September 1998. Downloaded from CLINICAL REVIEW Medical Consultation for the Elderly Patient With Hip Fracture RichardJ Ackermann, MD Background: This article describes a family physician geriatrician's perspective on the comprehensive management of hip fracture in frail elderly patients. Primary care physicians might be called upon to pro vide medical consultation for these patients. Methods: Guidelines were developed by a combination of personal experience in consulting for several hun dred elderly patients with hip fracture at a large community hospital, literature review using the key words "hip fractures," "aged," and "aged, 80 and over," and educational presentations for family practice residents. Results and Conclusions: Elderly patients with hip fracture offer a prime opportunity for comprehensive geriatric assessment. Intertrochanteric fractures are almost always treated with internal fixation, whereas femoral neck fractures can be treated by either fixation or by hemiarthroplasty. Hip fracture should be re garded as a surgical urgency, and generally operation should not be delayed, even if patients have serious comorbidity. The family physician can be instrumental in preparing the patient for surgery, preventing and treating complications, and assisting in the placement and rehabilitation of patients after hospital dis charge. 0 Am Board Fam Pract 1998; 11:366-77.) As the result of an aging population, family physi Some hip fractures and the falls that precede cians are increasingly likely to participate in the them are probably preventable. Strategies to de care of elderly patients suffering hip fracture. This tect and treat osteoporosis, especially in high-risk copyright. -

What You Should Know About Hip Fractures
Information O from Your Family Doctor What You Should Know About Hip Fractures What is a hip fracture? surgery. It may involve putting pins, rods, and A hip fracture is a break in the top of your plates into the hip joint. Some hip fractures are upper leg bone near the hip joint, just below treated with a hip replacement. The orthopedic the waist. The type of hip fracture depends surgeon will help decide which surgery is best on which part of the bone breaks. Most hip for you. fractures are caused by a fall in people 65 years or older. People with weak bones, known as What happens next? osteoporosis (OSS-tee-oh-puh-RO-sis), are You will need to work with a physical therapist more likely to break a hip. at home, in the therapist’s office, or in a skilled nursing facility to regain use of your hip. You will What are the symptoms? practice bending, walking, and climbing stairs. The most common symptom is pain in the hip For most patients, your doctor will or groin area. The pain is usually worse when recommend a medicine called a bisphosphonate you try to move the hip. There is a lot of pain (bis-FOSS-fuh-nate). This is taken by mouth. when you walk. Most people cannot walk with It can help lower your chance of another hip a hip fracture. fracture. How is it found? How can hip fractures be prevented? An x-ray can show if the hip is broken and You can prevent falls by talking to your doctor which part of the bone is fractured. -

Spinal Fractures Classification System an Aospine Knowledge Forum Initiative
Spinal Fractures Classification System an AOSpine Knowledge Forum initiative Subaxial Spine Fractures Thoracolumbar Spine Fractures Sacral Spine Fractures AOSpine–the leading global academic community for innovative education and research in spine care, inspiring lifelong learning and improving patients’ lives. Spinal Fractures Classification System 2 Spinal Fractures Classification System an AOSpine Knowledge Forum initiative CONTENT AOSpine Classification and Injury Severity System ................ 04 for Traumatic Fractures of the Subaxial Spine AOSpine Classification and Injury Severity System ................. 37 for Traumatic Fractures of the Thoracolumbar Spine AOSpine Classification and Injury Severity System .................55 for Traumatic Fractures of the Sacral Spine Spinal Fractures Classification System 3 AOSpine Knowledge Forum AOSpine Classification and Injury Severity System for Traumatic Fractures of the Subaxial Spine This is the present form of the classification the AOSpine Knowledge Forum (KF) SCI & Trauma is working on. It is the aim of the KF to develop a system, which can in the future be used as a tool for scientific research and a guide for treatment. This system is being subjected to a rigorous scientific assessment. Project members Aarabi B, Bellabarba C, Chapman J, Dvorak M, Fehlings M, Kandziora F, Kepler C, (in alphabetic order) Oner C, Rajasekaran S, Reinhold M, Schnake K, Vialle L and Vaccaro A. Disclaimer 1. Vaccaro, A. R., J. D. Koerner, K. E. Radcliff, F. C. Oner, M. Reinhold, K. J. Schnake, F. Kandziora, M. G. Fehlings, M. F. Dvorak, B. Aarabi, S. Rajasekaran, G. D. Schroeder, C. K. Kepler and L. R. Vialle (2015). “AOSpine subaxial cervical spine injury classification system.” Eur Spine J. 2. International validation process to be completed in 2015. -
Modifier Chart
Modifier Chart 22 Increased procedural services 23 Unusual anesthesia Unrelated Evaluation & Management (E/M) services by the same 24 provider during postoperative period Significant, separately identifiable E/M service by the same physician 25 on the same day of the procedure or other service 26 Professional component 32 Mandated Services When primary purpose is the delivery of an evidence-based service in accord with US Preventive Services Task Force A or B rating in effect 33 and other preventive services identified in preventive services mandates (legislative or regulatory) 47 Anesthesia by surgeon 50 Bilateral procedure 51 Multiple procedures 52 Reduced services 53 Discontinued procedure 54 Surgical care only 55 Postoperative management only 57 Decision for surgery Staged or related procedure or service by the same physician during 58 the postoperative period 59 Distinct procedural service Separate encounter, distinct because it occurred during a separate XE * encounter Separate structure, distinct because it was performed on a separate XS * organ/structure Separate practitioner, distinct because it was performed by a XP * different practitioner Unusual non-overlapping service, distinct because it does not XL * overlap usual components of the main service 62 Two surgeons 63 Procedure performed on infants less than 4 kgs. 66 Surgical team Repeat procedure or service by the same physician or other qualified 76 health care professional Repeat procedure or service by another physician or other qualified 77 health care professional Unplanned -

ACR Appropriateness Criteria Acute Hip Pain-Suspected Fracture
Revised 2018 American College of Radiology ACR Appropriateness Criteria® Acute Hip Pain-Suspected Fracture Variant 1: Acute hip pain. Fall or minor trauma. Suspect fracture. Initial imaging. Procedure Appropriateness Category Relative Radiation Level Radiography hip Usually Appropriate ☢☢☢ Radiography pelvis Usually Appropriate ☢☢ Radiography pelvis and hips Usually Appropriate ☢☢☢ CT pelvis and hips with IV contrast Usually Not Appropriate ☢☢☢ CT pelvis and hips without and with IV Usually Not Appropriate contrast ☢☢☢☢ CT pelvis and hips without IV contrast Usually Not Appropriate ☢☢☢ MRI pelvis and affected hip without and Usually Not Appropriate with IV contrast O MRI pelvis and affected hip without IV Usually Not Appropriate contrast O Bone scan hips Usually Not Appropriate ☢☢☢ US hip Usually Not Appropriate O Variant 2: Acute hip pain. Fall or minor trauma. Negative radiographs. Suspect fracture. Next imaging study. Procedure Appropriateness Category Relative Radiation Level MRI pelvis and affected hip without IV Usually Appropriate contrast O CT pelvis and hips without IV contrast Usually Appropriate ☢☢☢ CT pelvis and hips with IV contrast Usually Not Appropriate ☢☢☢ CT pelvis and hips without and with IV Usually Not Appropriate contrast ☢☢☢☢ MRI pelvis and affected hip without and with Usually Not Appropriate IV contrast O Bone scan hips Usually Not Appropriate ☢☢☢ US hip Usually Not Appropriate O ACR Appropriateness Criteria® 1 Acute Hip Pain-Suspected Fracture Acute Hip Pain-Suspected Fracture Expert Panel on Musculoskeletal Imaging: Andrew B. Ross, MD, MPHa; Kenneth S. Lee, MD, MBAb; Eric Y. Chang, MDc; Behrang Amini, MD, PhDd; Jennifer K. Bussell, MDe; Tetyana Gorbachova, MDf; Alice S. Ha, MDg; Bharti Khurana, MDh; Alan Klitzke, MDi; Pekka A. -

Lower Extremity Fracture Eponyms (Part 2) Philip Kin-Wai Wong1, Tarek N Hanna2*, Waqas Shuaib3, Stephen M Sanders4 and Faisal Khosa2
Wong et al. International Journal of Emergency Medicine (2015) 8:25 DOI 10.1186/s12245-015-0076-1 REVIEW Open Access What’s in a name? Lower extremity fracture eponyms (Part 2) Philip Kin-Wai Wong1, Tarek N Hanna2*, Waqas Shuaib3, Stephen M Sanders4 and Faisal Khosa2 Abstract Eponymous extremity fractures are commonly encountered in the emergency setting. Correct eponym usage allows rapid, succinct communication of complex injuries. We review both common and less frequently encountered extremity fracture eponyms, focusing on imaging features to identify and differentiate these injuries. We focus on plain radiographic findings, with supporting computed tomography (CT) images. For each injury, important radiologic descriptors are discussed which may need to be communicated to clinicians. Aspects of management and follow-up imaging recommendations are included. This is a two-part review: Part 1 focuses on fracture eponyms of the upper extremity, while Part 2 encompasses fracture eponyms of the lower extremity. Keywords: Eponyms; Fractures; Lower extremities; Imaging Introduction Review: Lower extremity fracture eponyms Eponyms are embedded throughout medicine; they Pipkin fracture can be found in medical literature, textbooks, and Femoral head fractures are relatively uncommon and even mass media. Their use allows physicians to are typically associated with hip dislocations after se- quickly provide a concise description of a complex vere high-impact trauma such as a motor vehicle colli- injury pattern. Eponymous extremity fractures are sion. Femoral head fractures are commonly grouped commonly encountered in the emergency setting and into the Pipkin classification (see Table 1) after the work are frequently used in interactions amongst radiolo- of the orthopedic surgeon Garrett Pipkin in 1957 (Fig.