Acer Menspessolanum Subsp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expression and Function of the Chloroplast-Encoded Gene Matk
Expression and Function of the Chloroplast-encoded Gene matK. Michelle Marie Barthet Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Biological Sciences K. W. Hilu, Chair E. Beers G. Gillaspy J. Sible R. A. Walker February 9, 2006 Blacksburg, Virginia Keywords: MatK, chloroplast, maturase, fast-evolving, Orchidaceae Copyright 2006, Michelle Marie Barthet Expression and Function of the chloroplast-encoded gene matK. Michelle Marie Barthet ABSTRACT The chloroplast matK gene has been identified as a rapidly evolving gene at nucleotide and corresponding amino acid levels. The high number of nucleotide substitutions and length mutations in matK has provided a strong phylogenetic signal for resolving plant phylogenies at various taxonomic levels. However, these same features have raised questions as to whether matK produces a functional protein product. matK is the only proposed chloroplast-encoded group II intron maturase. There are 15 genes in the chloroplast that would require a maturase for RNA splicing. Six of these genes have introns that are not excised by a nuclear imported maturase, leaving MatK as the only candidate for processing introns in these genes. Very little research has been conducted concerning the expression and function of this important gene and its protein product. It has become crucial to understand matK expression in light of its significance in RNA processing and plant systematics. In this study, we examined the expression, function and evolution of MatK using a combination of molecular and genetic methods. Our findings indicate that matK RNA and protein is expressed in a variety of plant species, and expression of MatK protein is regulated by development. -

Asterodiscus and Stigmatodiscus, Two New Apothecial Dothideomycete Genera and the New Order Stigmatodiscales
Fungal Diversity (2016) 80:271–284 DOI 10.1007/s13225-016-0356-y Asterodiscus and Stigmatodiscus, two new apothecial dothideomycete genera and the new order Stigmatodiscales Hermann Voglmayr1 & Alain Gardiennet 2 & Walter M. Jaklitsch1,3 Received: 25 August 2015 /Accepted: 11 January 2016 /Published online: 2 February 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract During a survey on corticolous Dothideomycetes, Keywords Ascomycota . Dothideomycetes . New species . several collections with ascospores matching the genera New genus . New family . New order . Phylogenetic analysis . Asteromassaria and Stigmatomassaria (Pleomassariaceae, Taxonomy Pleosporales) were revealed from dead corticated twigs of Acer, Carpinus and Tamarix. Closer morphological examina- tion showed that their ascomata were apothecial, with a Introduction hamathecium consisting of septate, branched paraphyses, which are apically swollen at maturity. Several collections During a survey on corticolous ascomycetes in Istria, Croatia were cultured and sequenced, and a Blast search of their nuc in September 2010, we collected a dothideomycetous species 28S rDNA sequences revealed dothideomycetous affiliation, from dead corticated twigs of Carpinus orientalis which but without a close match to a specific family or order. showed ascospores with a striking resemblance to Phylogenetic analyses of a multigene matrix containing a rep- Stigmatomassaria pupula (Pleomassariaceae, Pleosporales; resentative selection of Dothideomycetes -
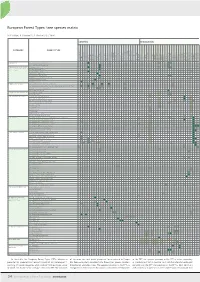
34 European Forest Types: Tree Species Matrix
M. Pividori, F. Giannetti,A.Barbati,G.Chirici M. Pividori,F. European ForestTypes:treespeciesmatrix of whicharedivided intosub-types.Forevery EFTthepresence types,some counting 14broadcategorieswhich include78forest presented as proposed and revised by Barbati andcolleagues presented asproposedandrevised by 34 coniferous forest forest coniferous andmixedbroadleaved- coniferous andnemoral 2. Hemiborealforest 1. Borealforest 14. Introducedtreespeciesforest aspen forest birch,or 13. Nonriverinealder, 12. Floodplainforest 11. Mireandswampforest Macaronesian regions Mediterranean, Anatolianand ofthe forests 10. Coniferous 9. Broadleavedevergreenforest 8. Thermophilousdeciduousforest 7. Mountainousbeechforest 6. Beechforest 5. Mesophyticdeciduousforest forest 4. Acidophilousoakandoak-birch forest 3. Alpineconiferous In this table the European Forest Types (EFTs) scheme is In thistabletheEuropeanForest Types(EFTs) CATEGORY 10.11 Mediterraneanyewstands articulatastands 10.10 Tetraclinis 10.9 Cedarforest 10.8 Cypressforest 10.7 Juniperforest 10.6 MediterraneanandAnatolianfirforest 10.5 Alti-Mediterraneanpineforest 10.4 MediterraneanandAnatolianScotspineforest 10.3 Canarianpineforest 10.2.2 AnatolianBlackpineforest 10.2.1 MediterraneanBlackpineforest -Pinuspinea 10.1.3 Mediterraneanpineforest -Pinushalepensis 10.1.2 Mediterraneanpineforest -Pinuspinaster 10.1.1 Mediterraneanpineforest 9.5 Othersclerophlyllousforests 9.4 Macaronesianlaurisilva groves 9.3 Palm 9.2 Olive-carobforest 9.1 Mediterraneanevergreenoakforest 8.8.8 Horsechestnutandwalnutmixedwoods -

Frawley Poster (NHRE 2016)
A Nuclear and Chloroplast Phylogeny of Maple Trees (Acer L.) and their close relatives (Hippocastanodeae, Sapindaceae) Emma Frawley1,2, AJ Harris2, Jun Wen2 1 Department of Environmental Studies, Bucknell University 2 Department of Botany, National Museum of Natural History INTRODUCTION: RESULTS AND DISCUSSION: Section Key: Acer carpinifolium Acer elegantulum A. Map Key: -/97Acer elegantulum B. Acer saccharum subsp. grandidentatum Acer pubipalmatum The primary goal of this study is to reconstruct a molecular phylogeny of the woody Palmata Acer elegantulum Acer pubipalmatum Acer hycranum Western North America Acer wuyangense Handeliodendron (Rehder) Acer wuyangense Handeliodendron Acer psuedosieboldianum Macrantha Acer campestre 99/100 Acer psuedosieboldianum trees and shrubs in Acer (L.), Dipteronia (Oliv.), the two members of the Acereae tribe, Acer miyabei subsp. miaotaiense Acer oliverianum 99 Acer oliverianum Rehder Platanoidea Acer saccharum subsp. floridatum Eastern North America Acer sp. - Hybrid AJ Harris Acer subsp. - US National Arboretum Acer sp. - Hybrid Acer sieboldianum and Aesculus (L.), Billia (Peyr.), and Handeliodendron (Rehder) of the Hippocastaneae Acer Acer diabolicum Acer sieboldianum Acer tataricum subsp. ginnala Acer sp. - Tibet Europe Acer sp. - Tibet Lithocarpa Acer tschonskii Acer sp. - Tibet Aesculus (L.) Acer pycnanthum Acer sp. - Tibet tribe. These five taxa make up the subfamily Hippocastanoideae in the family 98/100 Billia Peyr. Acer sacharinum Acer davidiiAcer davidii Ginnala Asia 98 Acer davidii Acer rubrum Acer davidii Sapindaceae. Acereae is especially interesting as it is a large, well-known, and Acer saccharum subsp. floridatum Acer crataegifolium Section Kevin Nixon Negundo 99/100Acer crataegifolium Acer griseum 99 Acer tegmentosum Acer triflorum Acer tegmentosum Trifoliata Acer triflorum 89/100 Acer miyabei subsp. -

Latin for Gardeners: Over 3,000 Plant Names Explained and Explored
L ATIN for GARDENERS ACANTHUS bear’s breeches Lorraine Harrison is the author of several books, including Inspiring Sussex Gardeners, The Shaker Book of the Garden, How to Read Gardens, and A Potted History of Vegetables: A Kitchen Cornucopia. The University of Chicago Press, Chicago 60637 © 2012 Quid Publishing Conceived, designed and produced by Quid Publishing Level 4, Sheridan House 114 Western Road Hove BN3 1DD England Designed by Lindsey Johns All rights reserved. Published 2012. Printed in China 22 21 20 19 18 17 16 15 14 13 1 2 3 4 5 ISBN-13: 978-0-226-00919-3 (cloth) ISBN-13: 978-0-226-00922-3 (e-book) Library of Congress Cataloging-in-Publication Data Harrison, Lorraine. Latin for gardeners : over 3,000 plant names explained and explored / Lorraine Harrison. pages ; cm ISBN 978-0-226-00919-3 (cloth : alkaline paper) — ISBN (invalid) 978-0-226-00922-3 (e-book) 1. Latin language—Etymology—Names—Dictionaries. 2. Latin language—Technical Latin—Dictionaries. 3. Plants—Nomenclature—Dictionaries—Latin. 4. Plants—History. I. Title. PA2387.H37 2012 580.1’4—dc23 2012020837 ∞ This paper meets the requirements of ANSI/NISO Z39.48-1992 (Permanence of Paper). L ATIN for GARDENERS Over 3,000 Plant Names Explained and Explored LORRAINE HARRISON The University of Chicago Press Contents Preface 6 How to Use This Book 8 A Short History of Botanical Latin 9 Jasminum, Botanical Latin for Beginners 10 jasmine (p. 116) An Introduction to the A–Z Listings 13 THE A-Z LISTINGS OF LatIN PlaNT NAMES A from a- to azureus 14 B from babylonicus to byzantinus 37 C from cacaliifolius to cytisoides 45 D from dactyliferus to dyerianum 69 E from e- to eyriesii 79 F from fabaceus to futilis 85 G from gaditanus to gymnocarpus 94 H from haastii to hystrix 102 I from ibericus to ixocarpus 109 J from jacobaeus to juvenilis 115 K from kamtschaticus to kurdicus 117 L from labiatus to lysimachioides 118 Tropaeolum majus, M from macedonicus to myrtifolius 129 nasturtium (p. -

The Red List of Revised and Extended
AcerThe Red List of revised and extended Dan Crowley, Megan Barstow, Malin Rivers & Yvette Harvey-Brown BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) is the world’s largest plant conservation network, comprising more than 500 botanic gardens in over 100 countries, and provides the secretariat to the IUCN/SSC Global Tree Specialist Group. BGCI was established in 1987 and is a registered charity with offices in the UK, US, China and Kenya. Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK. THE IUCN/SSC GLOBAL TREE SPECIALIST GROUP (GTSG) © 2020 Botanic Gardens Conservation International forms part of the Species Survival Commission’s network of over 7,000 ISBN-10: 10: 1-905164-73-4 volunteers working to stop the loss of plants, animals and their habitats. ISBN-13: 978-1-905164-73-8 SSC is the largest of the six Commissions of IUCN – The International Reproduction of any part of the publication for Union for Conservation of Nature. It serves as the main source of advice educational, conservation and other non-profit to the Union and its members on the technical aspects of species purposes is authorized without prior permission from conservation. The aims of the IUCN/SSC Global Tree Specialist Group the copyright holder, provided that the source is fully acknowledged. are to promote and implement global red listing for trees and to act in an advisory capacity to the Global Trees Campaign. Reproduction for resale or other commercial purposes is prohibited without prior written permission from the copyright holder. Recommended citation: Crowley, D., Barstow, M., Rivers, M. -
Geographic Variations of the Wood Density and Fiber Dimensions of the Persian Oak Wood
Article Geographic Variations of the Wood Density and Fiber Dimensions of the Persian Oak Wood Noorollah Nazari 1, Mohsen Bahmani 1,*, Saleh Kahyani 1, Miha Humar 2 and Gerald Koch 3 1 Department of Natural Resources and Earth Science, Shahrekord University, Shahrekord 64165478, Iran; [email protected] (N.N.); [email protected] (S.K.) 2 Department of Wood Science, Biotechnical Faculty, University of Ljubljana, 1501 Ljubljana, Slovenia; [email protected] 3 Thünen Institute of Wood Research Hamburg, Leuschnerstraße 91, 21031 Hamburg-Bergedorf, Germany; [email protected] * Correspondence: [email protected]; Tel.: +98-383-2324-401 Received: 27 July 2020; Accepted: 12 September 2020; Published: 17 September 2020 Abstract: Persian oak (Quercus brantii Lindl.) is a valuable native species in Iranian forests with very limited availability of data on its wood properties. The objective of the current study was to determine the influence of altitude and slope on physical properties and fiber dimensions of Persian oak wood. In addition, the relationship among wood properties, site conditions (temperature and rainfall) and growth traits of trees (tree height, DBH, basal area, age, crown diameter, crown basal area, volume and annual diameter increment) were studied by principal component analysis (PCA). Three altitude levels (1730, 1980 and 2250 m) and three slope classes (<30%, 30–45% and >45%) were considered in the current study. It was determined that trees growing in the intermediate altitude (1980 m) showed the highest oven-dry density values, and those in the lowest altitude (1730 m) revealed the lowest ones. The results also indicate significant statistical differences between altitude levels and slope classes on the fiber length, fiber diameter and volumetric swelling at the 99% confidence interval while no significant differences were found between average values of oven-dry density among different altitudes and slopes. -

Eulogy for Acer
Eulogy for Acer ... For an all too brief period each autumn the woods of North America and the Orient, and many gardens throughout the temperate world, are ablaze with scarlet, gold, and yellow when maples, the most spectacular of all trees, adorn the countryside. Maples delight the eye, provide one of nature's finest sweets, and hold their own as timber with even the mighty oaks. Throughout the ages people have been enchanted with this magnificent and versatile genus of trees known as Acer. (Oterdoom 1994 : 15) (Hajime Hayashida) Morphology and systematics of the genus Acer (Sapindaceae), incl. infrageneric classification morphology systematics genus Acer Sapindaceae IG classification Acer = ??? - a Taiwan-based computer company - a genus of trees and shrubs - Armored Combat Engineer Robot, by Mesa Robotics - Australian Council for Educational Research - David Acer, Canadese stand-up comedian Acer etymology ac- = pointed akros (Greek) = pointed acer (Latin) = sharp e.g. Acanthus Actinoscirpus = sedge with star-shaped inflorescence Acer = pointed leaves ? or : extremely hard wood ? (spears !) Classification BA APG II 2003 MC Angiosperm Phylogeny Group euDC Classification APG II 2003 Angiosperm Phylogeny Group order Sapindales 9 families 460 genera 5670 species trees with pinnate leaves noxious secondary metabolites (www.mobot.org/MOBOT/Research/APweb) Sapindaceae sensu lato : phylogeny area APWeb 2007 (after Harrington et al. 2005, Syst.Bot. 30) Genera in Sapindaceae Sapindus mukorossi Soapberry tree Dodonaea viscosa Hop bush Koelreuteria -

Acer Monspessulanum Ssp. Microphyllum (Montpellier Maple) Acer Monspessulanum Ssp
Acer monspessulanum ssp. microphyllum (Montpellier maple) Acer monspessulanum ssp. microphyllum is a medium sized tree native to Turkey and Lebanon that can reach up to 10 m high. Its young branches are often red in color. The leaves are small up to 3 cm long. It flowers in the Spring from March to May. This tree is found mostly at altitudes higher than 800 m and in natural reserves as Tannourine narture reserve. It can be sheared as a hedge and grows well in pots. It can also be grown as a Bonsai. Landscape Information ﻗﻴﻘﺐ ﺣﺮﻣﻮﻥ :Arabic Name Plant Type: Tree Origin: Turkey and Lebanon Heat Zones: Hardiness Zones: 6, 7, 8, 9 Uses: Hedge, Bonsai, Specimen, Container, Native to Lebanon Size/Shape Tree Shape: Round Canopy Symmetry: Symmetrical Canopy Density: Medium Plant Image Canopy Texture: Medium Height at Maturity: 5 to 8 m Spread at Maturity: 5 to 8 meters Acer monspessulanum ssp. microphyllum (Montpellier maple) Botanical Description Foliage Leaf Arrangement: Opposite Leaf Venation: Parallel Leaf Persistance: Deciduous Leaf Type: Trifoliate Leaf Blade: Less than 5 Leaf Shape: Star Leaf Margins: Entire Leaf Textures: Glossy Leaf Scent: No Fragance Color(growing season): Green Color(changing season): Yellow, Orange, Red Flower Flower Showiness: False Flower Size Range: 1.5 - 3 Flower Type: Corymb Flower Sexuality: Monoecious (Bisexual) Flower Scent: No Fragance Flower Color: Green, Yellow Seasons: Spring Fruit Flower Image Fruit Type: Samara Fruit Showiness: True Fruit Size Range: 3 - 7 Fruit Colors: Red Seasons: Spring Acer monspessulanum ssp. microphyllum (Montpellier maple) Horticulture Management Tolerance Frost Tolerant: Yes Heat Tolerant: Yes Drought Tolerant: Yes Salt Tolerance: Moderate Requirements Soil Requirements: Soil Ph Requirements: Water Requirements: Light Requirements: Full, Part, Shade Management Edible Parts: None Leaf Image Plant Propagations: Seed, Cutting MORE IMAGES Fruit Image. -
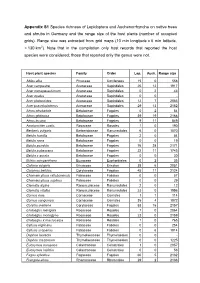
Appendix S1 Species Richness of Lepidoptera and Auchenorrhyncha on Native Trees and Shrubs in Germany and the Range Size of the Host Plants (Number of Occupied Grids)
Appendix S1 Species richness of Lepidoptera and Auchenorrhyncha on native trees and shrubs in Germany and the range size of the host plants (number of occupied grids). Range size was extracted from grid maps (10 min longitude x 6 min latitude, ≈ 130 km 2). Note that in the compilation only host records that reported the host species were considered; those that reported only the genus were not. Host plant species Family Order Lep. Auch. Range size Abies alba Pinaceae Coniferales 15 0 558 Acer campestre Aceraceae Sapindales 25 12 1917 Acer monspessulanum Aceraceae Sapindales 0 2 43 Acer opalus Aceraceae Sapindales 0 0 - Acer platanoides Aceraceae Sapindales 12 7 2083 Acer pseudoplatanus Aceraceae Sapindales 29 13 2152 Alnus alnobetula Betulaceae Fagales 0 2 54 Alnus glutinosa Betulaceae Fagales 39 19 2168 Alnus incana Betulaceae Fagales 9 11 849 Amelanchier ovalis Rosaceae Rosales 1 0 190 Berberis vulgaris Berberidaceae Ranunculales 6 0 1070 Betula humilis Betulaceae Fagales 2 0 54 Betula nana Betulaceae Fagales 0 0 19 Betula pendula Betulaceae Fagales 76 28 2171 Betula pubescens Betulaceae Fagales 22 11 1745 Betula x aurata Betulaceae Fagales 0 0 30 Buxus sempervirens Buxaceae Euphorbiales 0 2 35 Calluna vulgaris Ericaceae Ericales 38 6 2051 Carpinus betulus Corylaceae Fagales 45 11 2124 Chamaecytisus ratisbonensis Fabaceae Fabales 0 0 57 Chamaecytisus supinus Fabaceae Fabales 0 0 29 Clematis alpina Ranunculaceae Ranunculales 2 0 12 Clematis vitalba Ranunculaceae Ranunculales 23 0 1556 Cornus mas Cornaceae Cornales 1 1 114 Cornus sanguinea -

Thesis Multi-Site Trial of Woody
THESIS MULTI-SITE TRIAL OF WOODY PLANTS: 2006 PLANTING AND EVALUATION OF DIFFERENCES IN DROUGHT TOLERANCE OF THREE AMELANCHIER SPECIES Submitted by Eric Hammond Department of Horticulture and Landscape Architecture In partial fulfillment of the requirements For the Degree of Master of Science Colorado State University Fort Collins, Colorado Spring 2012 Master’s Committee: Advisor: James Klett William Jacobi Harrison Hughes Copyright by Eric Hammond 2012 All Rights Reserved ABSTRACT MULTI-SITE TRIAL OF WOODY PLANTS: 2006 PLANTING AND EVALUATION OF DIFFERENCES IN DROUGHT TOLERANCE OF THREE AMELANCHIER SPECIES CHAPTER 1: MULTI-SITE WOODY PLANT EVALUATION IN COLORADO A multi-site trial of several woody plants plant species and cultivars was conducted to determine their potential for landscape use in the state of Colorado. The trial was conducted with the input and cooperation for the Colorado Nursery Research and Education Foundation and Plant Select®. Data was collected from 2006-2010 at five sites with different soils, climates and cultural practices. Plants were evaluated based on size, growth, survival, aesthetics and heath. The taxa evaluated were: Acer monspessulanum, Juniperus scopulorum 'Woodward', Larix decidua, Prunus serotina (of central Texas providence), Pyrus ussuriensis 'Burgundy', Quercus polymorpha and, Quercus × pauciloba. Researchers recommend Juniperus scopulorum 'Woodward', Prunus serotina and, Quercus × pauciloba for widespread use in the state. Quercus polymorpha did not prove adapted to any of the sites and is not recommended. Larix decidua and Pyrus ussuriensis 'Burgundy' did not perform well at all sites and are only recommended for use in the state in some situations. ii CHAPTER 2: EVALUATION OF DIFFERENCES IN DROUGHT TOLERANCE OF THREE AMELANCHIER SPECIES In the summer and fall of 2010, research was conducted to evaluate the drought tolerance of Amelanchier alnifolia, Amelanchier canadensis, and Amelanchier utahensis. -

Urban Forest Resilience Through Tree Selection—Variation in Drought Tolerance in Acer
Urban Forestry & Urban Greening 14 (2015) 858–865 Contents lists available at ScienceDirect Urban Forestry & Urban Greening j ournal homepage: www.elsevier.com/locate/ufug Urban forest resilience through tree selection—Variation in drought tolerance in Acer a,∗ b c Henrik Sjöman , Andrew D. Hirons , Nina L. Bassuk a Swedish University of Agricultural Sciences, Faculty of Landscape Planning, Horticulture and Agricultural Science, Department of Landscape Architecture, Planning and Management, PO Box 66, SE-23053 Alnarp, Sweden b Myerscough College, Bilsborrow, Preston, Lancashire PR3 0RY, UK c Cornell University, College of Agriculture and Life Science, Department of Horticulture, 134A Plant Science Building, Ithaca, NY 14853-5904, USA a r a t i c l e i n f o b s t r a c t Article history: It is widely recognized that trees contribute a range of ecosystem services in urban environments. How- Received 6 March 2015 ever, the magnitude of their contribution is closely related to their physiological condition and capacity Received in revised form 19 July 2015 to persist within our towns and cities. Root loss during transplanting, limited soil volume, disruption to Accepted 8 August 2015 soil hydrological processes and impermeable surfaces result in water deficits being major physiological stress limiting the performance of urban trees. The leaf water potential at turgor loss (« P0) provides a Keywords: robust measure of drought tolerance since a more negative « P0 allows the leaf to maintain physiological Ecosystem services function over a wider range of leaf water potentials and, by implication, soil matric potentials (« soil). Leaf turgor loss point « Maples In this study, P0 was calculated for 27 maple (Acer) genotypes based on a known linear relationship between the osmotic potential at full turgor (« 100) and « P0.