A Study of the Characteristics of the Appearances of Lepidoptera Larvae and Foodplants at Mt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Frontiers in Zoology Biomed Central
Frontiers in Zoology BioMed Central Research Open Access Does the DNA barcoding gap exist? – a case study in blue butterflies (Lepidoptera: Lycaenidae) Martin Wiemers* and Konrad Fiedler Address: Department of Population Ecology, Faculty of Life Sciences, University of Vienna, Althanstrasse 14, 1090 Vienna, Austria Email: Martin Wiemers* - [email protected]; Konrad Fiedler - [email protected] * Corresponding author Published: 7 March 2007 Received: 1 December 2006 Accepted: 7 March 2007 Frontiers in Zoology 2007, 4:8 doi:10.1186/1742-9994-4-8 This article is available from: http://www.frontiersinzoology.com/content/4/1/8 © 2007 Wiemers and Fiedler; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: DNA barcoding, i.e. the use of a 648 bp section of the mitochondrial gene cytochrome c oxidase I, has recently been promoted as useful for the rapid identification and discovery of species. Its success is dependent either on the strength of the claim that interspecific variation exceeds intraspecific variation by one order of magnitude, thus establishing a "barcoding gap", or on the reciprocal monophyly of species. Results: We present an analysis of intra- and interspecific variation in the butterfly family Lycaenidae which includes a well-sampled clade (genus Agrodiaetus) with a peculiar characteristic: most of its members are karyologically differentiated from each other which facilitates the recognition of species as reproductively isolated units even in allopatric populations. -

Schutz Des Naturhaushaltes Vor Den Auswirkungen Der Anwendung Von Pflanzenschutzmitteln Aus Der Luft in Wäldern Und Im Weinbau
TEXTE 21/2017 Umweltforschungsplan des Bundesministeriums für Umwelt, Naturschutz, Bau und Reaktorsicherheit Forschungskennzahl 3714 67 406 0 UBA-FB 002461 Schutz des Naturhaushaltes vor den Auswirkungen der Anwendung von Pflanzenschutzmitteln aus der Luft in Wäldern und im Weinbau von Dr. Ingo Brunk, Thomas Sobczyk, Dr. Jörg Lorenz Technische Universität Dresden, Fakultät für Umweltwissenschaften, Institut für Forstbotanik und Forstzoologie, Tharandt Im Auftrag des Umweltbundesamtes Impressum Herausgeber: Umweltbundesamt Wörlitzer Platz 1 06844 Dessau-Roßlau Tel: +49 340-2103-0 Fax: +49 340-2103-2285 [email protected] Internet: www.umweltbundesamt.de /umweltbundesamt.de /umweltbundesamt Durchführung der Studie: Technische Universität Dresden, Fakultät für Umweltwissenschaften, Institut für Forstbotanik und Forstzoologie, Professur für Forstzoologie, Prof. Dr. Mechthild Roth Pienner Straße 7 (Cotta-Bau), 01737 Tharandt Abschlussdatum: Januar 2017 Redaktion: Fachgebiet IV 1.3 Pflanzenschutz Dr. Mareike Güth, Dr. Daniela Felsmann Publikationen als pdf: http://www.umweltbundesamt.de/publikationen ISSN 1862-4359 Dessau-Roßlau, März 2017 Das diesem Bericht zu Grunde liegende Vorhaben wurde mit Mitteln des Bundesministeriums für Umwelt, Naturschutz, Bau und Reaktorsicherheit unter der Forschungskennzahl 3714 67 406 0 gefördert. Die Verantwortung für den Inhalt dieser Veröffentlichung liegt bei den Autorinnen und Autoren. UBA Texte Entwicklung geeigneter Risikominimierungsansätze für die Luftausbringung von PSM Kurzbeschreibung Die Bekämpfung -

Xyleninae 73.087 2385 Small Mottled Willow
Xyleninae 73.087 2385 Small Mottled Willow (Spodoptera exigua) 73.089 2386 Mediterranean Brocade (Spodoptera littoralis) 73.091 2396 Rosy Marbled (Elaphria venustula) 73.092 2387 Mottled Rustic (Caradrina morpheus) 73.093 2387a Clancy's Rustic (Caradrina kadenii) 73.095 2389 Pale Mottled Willow (Caradrina clavipalpis) 73.096 2381 Uncertain (Hoplodrina octogenaria) 73.0961 2381x Uncertain/Rustic agg. (Hoplodrina octogenaria/blanda) 73.097 2382 Rustic (Hoplodrina blanda) 73.099 2384 Vine's Rustic (Hoplodrina ambigua) 73.100 2391 Silky Wainscot (Chilodes maritima) 73.101 2380 Treble Lines (Charanyca trigrammica) 73.102 2302 Brown Rustic (Rusina ferruginea) 73.103 2392 Marsh Moth (Athetis pallustris) 73.104 2392a Porter's Rustic (Athetis hospes) 73.105 2301 Bird's Wing (Dypterygia scabriuscula) 73.106 2304 Orache Moth (Trachea atriplicis) 73.107 2300 Old Lady (Mormo maura) 73.109 2303 Straw Underwing (Thalpophila matura) 73.111 2097 Purple Cloud (Actinotia polyodon) 73.113 2306 Angle Shades (Phlogophora meticulosa) 73.114 2305 Small Angle Shades (Euplexia lucipara) 73.118 2367 Haworth's Minor (Celaena haworthii) 73.119 2368 Crescent (Helotropha leucostigma) 73.120 2352 Dusky Sallow (Eremobia ochroleuca) 73.121 2364 Frosted Orange (Gortyna flavago) 73.123 2361 Rosy Rustic (Hydraecia micacea) 73.124 2362 Butterbur (Hydraecia petasitis) 73.126 2358 Saltern Ear (Amphipoea fucosa) 73.127 2357 Large Ear (Amphipoea lucens) 73.128 2360 Ear Moth (Amphipoea oculea) 73.1281 2360x Ear Moth agg. (Amphipoea oculea agg.) 73.131 2353 Flounced Rustic (Luperina -

Noctuid Moth (Lepidoptera, Noctuidae) Communities in Urban Parks of Warsaw
POLISH ACADEMY OF SCIENCES • INSTITUTE OF ZOOLOGY MEMORABILIA ZOOLOGICA MEMORABILIA ZOOL. 42 125-148 1986 GRAŻYNA WINIARSKA NOCTUID MOTH (LEPIDOPTERA, NOCTUIDAE) COMMUNITIES IN URBAN PARKS OF WARSAW ABSTRACT A total of 40 noctuid moth species were recorded in four parks of Warsaw. Respective moth communities consisted of a similar number of species (17—25), but differed in their abundance index (3.5 —7.9). In all the parks, the dominant species were Autographa gamma and Discrestra trifolii. The subdominant species were represented by Acronicta psi, Trachea atriplicis, Mamestra suasa, Mythimna pallens, and Catocala nupta. There were differences in the species composition and dominance structure among noctuid moth communities in urban parks, suburban linden- oak-hornbeam forest, and natural linden-oak-hornbeam forest. In the suburban and natural linden-oak-hornbeam forests, the number of species was higher by 40% and their abundance wao 5 — 9 times higher than in the urban parks. The species predominating in parks occurred in very low numbers in suburban and natural habitats. Only T. atriplicis belonged to the group of most abundant species in all the habitats under study. INTRODUCTION In recent years, the interest of ecologists in urban habitats has been increasing as they proved to be rich in plant and animal species. The vegetation of urban green areas is sufficiently well known since its species composition and spatial structure are shaped by gardening treatment. But the fauna of these areas is poorly known, and regular zoological investigations in urban green areas were started not so long ago, when urban green was recognized as one of the most important factors of the urban “natural” habitat (Ciborowski 1976). -

Lepidoptera, Tortricidae) from Mt
Accepted Manuscript Tortricinae (Lepidoptera, Tortricidae) from Mt. Changbai-shan, China Kyu-Tek Park, Bong-Woo Lee, Yang-Seop Bae, Hui-Lin Han, Bong-Kyu Byun PII: S2287-884X(14)00025-9 DOI: 10.1016/j.japb.2014.04.007 Reference: JAPB 19 To appear in: Journal of Asia-Pacific Biodiversity Received Date: 28 February 2014 Revised Date: 13 March 2014 Accepted Date: 4 April 2014 Please cite this article as: Park K-T, Lee B-W, Bae Y-S, Han H-L, Byun B-K, Tortricinae (Lepidoptera, Tortricidae) from Mt. Changbai-shan, China, Journal of Asia-Pacific Biodiversity (2014), doi: 10.1016/ j.japb.2014.04.007. This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT J. of Asia-Pacific Biodiversity Tortricinae (Lepidoptera, Tortricidae) from Mt. Changbai-shan, China Kyu-Tek Park a, Bong-Woo Lee b, Yang-Seop Bae c, Hui-Lin Han d, Bong-Kyu Byun e* a The Korean Academy of Science and Technology, Seongnam, 463-808, Korea b Division of Forest Biodiversity, Korea National Arboretum, Sumokwokgil, Pocheon, 487-821, Korea c Division of Life Sciences, University of Incheon, 12-1 Songdo-dong, Yeonsu-gu, Incheon, 406-772, Korea dSchool of Forestry, Northeast Forestry University, Harbin, 150040, P.R. -

Lepidoptera: Tortricidae: Tortricinae) and Evolutionary Correlates of Novel Secondary Sexual Structures
Zootaxa 3729 (1): 001–062 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Monograph ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3729.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:CA0C1355-FF3E-4C67-8F48-544B2166AF2A ZOOTAXA 3729 Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures JASON J. DOMBROSKIE1,2,3 & FELIX A. H. SPERLING2 1Cornell University, Comstock Hall, Department of Entomology, Ithaca, NY, USA, 14853-2601. E-mail: [email protected] 2Department of Biological Sciences, University of Alberta, Edmonton, Canada, T6G 2E9 3Corresponding author Magnolia Press Auckland, New Zealand Accepted by J. Brown: 2 Sept. 2013; published: 25 Oct. 2013 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 JASON J. DOMBROSKIE & FELIX A. H. SPERLING Phylogeny of the tribe Archipini (Lepidoptera: Tortricidae: Tortricinae) and evolutionary correlates of novel secondary sexual structures (Zootaxa 3729) 62 pp.; 30 cm. 25 Oct. 2013 ISBN 978-1-77557-288-6 (paperback) ISBN 978-1-77557-289-3 (Online edition) FIRST PUBLISHED IN 2013 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2013 Magnolia Press 2 · Zootaxa 3729 (1) © 2013 Magnolia Press DOMBROSKIE & SPERLING Table of contents Abstract . 3 Material and methods . 6 Results . 18 Discussion . 23 Conclusions . 33 Acknowledgements . 33 Literature cited . 34 APPENDIX 1. 38 APPENDIX 2. 44 Additional References for Appendices 1 & 2 . 49 APPENDIX 3. 51 APPENDIX 4. 52 APPENDIX 5. -
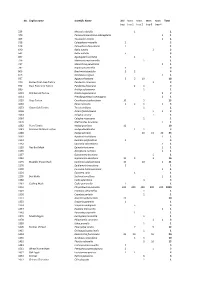
Full List Here
No. English name Scientific Name JBM Mark Mark Mark Mark Total trap trap 1 trap 2 trap 3 trap 4 229 Monopis obviella 1 1 440 Paraswammerdamia albicapitella 1 1 463 Ypsolopha vittella 1 1 518 Coleophora mayrella 2 2 519 Coleophora deauratella 1 1 640 Batia lunaris 2 2 642 Batia unitella 5 5 697 Agonopterix arenella 1 1 726 Metzneria metzneriella 1 1 737 Monochroa palustrella 1 1 787 Bryotropha terrella 3 3 866 Brachmia blandella 2 2 4 873 Blastobasis lignea 1 1 937 Agapeta hamana 3 5 10 18 970 Barred Fruit-tree Tortrix Pandemis cerasana 2 2 972 Dark Fruit-tree Tortrix Pandemis heparana 2 1 3 980 Archips xylosteana 1 1 1010 Red-barred Tortrix Ditula angustiorana 2 2 1011 Pseudargyrotoza conwagana 1 1 1020 Grey Tortrix Cnephasia stephensiana 10 5 15 1030 Eana incanana 1 2 1 1 5 1033 Green Oak Tortrix Tortrix viridana 1 1 1036 Acleris forsskaleana 1 1 2 1063 Celypha striana 1 1 1064 Celypha rosaceana 1 1 1076 Olethreutes lacunana 1 1 1082 Plum Tortrix Hedya pruniana 10 10 1083 Marbled Orchard Tortrix Hedya dimidioalba 1 1 2 1086 Hedya salicella 10 15 20 45 1092 Apotomis turbidana 1 1 1113 Eudemis profundana 5 5 1132 Eponotia subocellana 1 1 1139 Nut Bud Moth Epinotia tenerana 1 1 1165 Zeiraphera isertana 1 1 1167 Gypsonoma aceriana 1 1 1169 Gypsonoma dealbana 10 3 3 16 1175 Bramble Shoot Moth Epiblema uddmanniana 10 10 1 21 1176 Epiblema trimaculana 1 1 1200 Eucosma hohenwartiana 1 1 1201 Eucosma cana 1 1 1205 Bud Moth Spilonota ocellana 1 1 1260 Cydia splendana 3 3 1261 Codling Moth Cydia pomonella 1 1 1293 Chrysoteuchia culmella 200 200 -

Diptera) of the Czech Republic
© Entomologica Fennica. 30 March 2009 Annotated host catalogue for the Tachinidae (Diptera) of the Czech Republic Jaromir Vafihara*, Hans-Peter Tschorsnig, Benno Herting’r, Petr Mfickstein & Veronika Michalkova J P. & V. Vanhara, ., Tschorsnig, H.-P., Herting, B., Miickstein, Michalkova, 2009: Annotated host catalogue for the Tachinidae (Diptera) of the Czech Re- public. — Entomol. Fennica 20: 22—48. An annotated host catalogue is given for the Tachinidae ofthe Czech Republic. It comprises 149 of476 tachinid species which are currently known from this coun- try (included the two new records cited below). 195 hosts are listed. The first host records ofTachinidae date back to the second halfofthe 19th century. The bibli- ography for the host records consists of 1 16 papers of 55 researchers. Several re- cords of hitherto unpublished material are included. Phryxe setifacies and Anthomyiopsis plagioderae are first records for the Czech Republic. J. Vanhara (*corresponding author), Masaryk University, Faculty ofScience, Kotlarska 2, CZ—6I I 3 7 Brno, Czech Republic, [email protected] H.—P. Tschorsnig, Staatliches Museumflir Naturkunde, Rosenstein I, D— 70 191 Stuttgart, Germany, tschorsnig.smns@naturkundemuseum—bw.de P. Muckstein Administration of the Protected Landscape Area Zd’drske' vrchy, Brnenska 39, CZ—591 01 Zd’dr nad Sazavou, Czech Republic, muchstein @email.cz V. Michalkova, Masaryk University, Faculty ofScience, Kotlarska 2, CZ—6I I 3 7 Brno, Czech Republic, [email protected] Received 22 August 200 7, accepted 21 January 2008 1. Introduction The tachinid species are listed in their actual valid nomenclature; probable misidentifications Tachinidae are a very large and important dipter- are — if possible — tentatively corrected, but the an family of (mainly) insect parasitoids. -

Description and Phylogenetic Analysis of the Calycopidina (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): a Subtribe of Detritivores
Description and phylogenetic analysis of the Calycopidina 45 Description and phylogenetic analysis of the Calycopidina (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): a subtribe of detritivores Marcelo Duarte1 & Robert K. Robbins2 1Museu de Zoologia, Universidade de São Paulo, Avenida Nazaré 481, Ipiranga, 04263–000 São Paulo, SP, Brazil. [email protected] 2National Museum of Natural History, Smithsonian Institution, P. O. Box 37012, NHB Stop 105, Washington, DC 20013–7012 USA. [email protected] ABSTRACT. Description and phylogenetic analysis of the Calycopidina (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): a subtribe of detritivores. The purpose of this paper is to establish a phylogenetic basis for a new Eumaeini subtribe that includes those lycaenid genera in which detritivory has been recorded. Morphological characters were coded for 82 species of the previously proposed “Lamprospilus Section” of the Eumaeini (19 of these had coding identical to another species), and a phylogenetic analysis was performed using the 63 distinct ingroup terminal taxa and six outgroups belonging to four genera. Taxonomic results include the description in the Eumaeini of Calycopidina Duarte & Robbins new subtribe (type genus Calycopis Scudder, 1876), which contains Lamprospilus Geyer, Badecla Duarte & Robbins new genus (type species Thecla badaca Hewitson), Arzecla Duarte & Robbins new genus (type species Thecla arza Hewitson), Arumecla Robbins & Duarte, Camissecla Robbins & Duarte, Electrostrymon Clench, Rubroserrata K. Johnson & Kroenlein revalidated status, Ziegleria K. Johnson, Kisutam K. Johnson & Kroenlein revalidated status, and Calycopis. Previous “infratribe” names Angulopina K. Johnson & Kroenlein, 1993, and Calycopina K. Johnson & Kroenlein, 1993, are nomenclaturally unavailable and polyphyletic as proposed. New combinations include Badecla badaca (Hewitson), Badecla picentia (Hewitson), Badecla quadramacula (Austin & K. -

The Entomologist's Record and Journal of Variation
M DC, — _ CO ^. E CO iliSNrNVINOSHilWS' S3ldVyan~LIBRARlES*"SMITHS0N!AN~lNSTITUTl0N N' oCO z to Z (/>*Z COZ ^RIES SMITHSONIAN_INSTITUTlON NOIiniIiSNI_NVINOSHllWS S3ldVaan_L: iiiSNi'^NviNOSHiiNS S3iavyan libraries Smithsonian institution N( — > Z r- 2 r" Z 2to LI ^R I ES^'SMITHSONIAN INSTITUTlON'"NOIini!iSNI~NVINOSHilVMS' S3 I b VM 8 11 w </» z z z n g ^^ liiiSNi NviNOSHims S3iyvyan libraries Smithsonian institution N' 2><^ =: to =: t/J t/i </> Z _J Z -I ARIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS SSIdVyan L — — </> — to >'. ± CO uiiSNi NViNosHiiws S3iyvaan libraries Smithsonian institution n CO <fi Z "ZL ~,f. 2 .V ^ oCO 0r Vo^^c>/ - -^^r- - 2 ^ > ^^^^— i ^ > CO z to * z to * z ARIES SMITHSONIAN INSTITUTION NOIinillSNl NVINOSHllWS S3iaVdan L to 2 ^ '^ ^ z "^ O v.- - NiOmst^liS^> Q Z * -J Z I ID DAD I re CH^ITUCnMIAM IMOTtTIITinM / c. — t" — (/) \ Z fj. Nl NVINOSHIIINS S3 I M Vd I 8 H L B R AR I ES, SMITHSONlAN~INSTITUTION NOIlfl :S^SMITHS0NIAN_ INSTITUTION N0liniliSNI__NIVIN0SHillMs'^S3 I 8 VM 8 nf LI B R, ^Jl"!NVINOSHimS^S3iavyan"'LIBRARIES^SMITHS0NIAN~'lNSTITUTI0N^NOIin L '~^' ^ [I ^ d 2 OJ .^ . ° /<SS^ CD /<dSi^ 2 .^^^. ro /l^2l^!^ 2 /<^ > ^'^^ ^ ..... ^ - m x^^osvAVix ^' m S SMITHSONIAN INSTITUTION — NOIlfliliSNrNVINOSHimS^SS iyvyan~LIBR/ S "^ ^ ^ c/> z 2 O _ Xto Iz JI_NVIN0SH1I1/MS^S3 I a Vd a n^LI B RAR I ES'^SMITHSONIAN JNSTITUTION "^NOlin Z -I 2 _j 2 _j S SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVaan LI BR/ 2: r- — 2 r- z NVINOSHiltNS ^1 S3 I MVy I 8 n~L B R AR I Es'^SMITHSONIAN'iNSTITUTIOn'^ NOlin ^^^>^ CO z w • z i ^^ > ^ s smithsonian_institution NoiiniiiSNi to NviNosHiiws'^ss I dVH a n^Li br; <n / .* -5^ \^A DO « ^\t PUBLISHED BI-MONTHLY ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. -

Diversity of the Moth Fauna (Lepidoptera: Heterocera) of a Wetland Forest: a Case Study from Motovun Forest, Istria, Croatia
PERIODICUM BIOLOGORUM UDC 57:61 VOL. 117, No 3, 399–414, 2015 CODEN PDBIAD DOI: 10.18054/pb.2015.117.3.2945 ISSN 0031-5362 original research article Diversity of the moth fauna (Lepidoptera: Heterocera) of a wetland forest: A case study from Motovun forest, Istria, Croatia Abstract TONI KOREN1 KAJA VUKOTIĆ2 Background and Purpose: The Motovun forest located in the Mirna MITJA ČRNE3 river valley, central Istria, Croatia is one of the last lowland floodplain 1 Croatian Herpetological Society – Hyla, forests remaining in the Mediterranean area. Lipovac I. n. 7, 10000 Zagreb Materials and Methods: Between 2011 and 2014 lepidopterological 2 Biodiva – Conservation Biologist Society, research was carried out on 14 sampling sites in the area of Motovun forest. Kettejeva 1, 6000 Koper, Slovenia The moth fauna was surveyed using standard light traps tents. 3 Biodiva – Conservation Biologist Society, Results and Conclusions: Altogether 403 moth species were recorded Kettejeva 1, 6000 Koper, Slovenia in the area, of which 65 can be considered at least partially hygrophilous. These results list the Motovun forest as one of the best surveyed regions in Correspondence: Toni Koren Croatia in respect of the moth fauna. The current study is the first of its kind [email protected] for the area and an important contribution to the knowledge of moth fauna of the Istria region, and also for Croatia in general. Key words: floodplain forest, wetland moth species INTRODUCTION uring the past 150 years, over 300 papers concerning the moths Dand butterflies of Croatia have been published (e.g. 1, 2, 3, 4, 5, 6, 7, 8). -

Specimen Records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895
Catalog: Oregon State Arthropod Collection 2019 Vol 3(2) Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895 Jon H. Shepard Paul C. Hammond Christopher J. Marshall Oregon State Arthropod Collection, Department of Integrative Biology, Oregon State University, Corvallis OR 97331 Cite this work, including the attached dataset, as: Shepard, J. S, P. C. Hammond, C. J. Marshall. 2019. Specimen records for North American Lepidoptera (Insecta) in the Oregon State Arthropod Collection. Lycaenidae Leach, 1815 and Riodinidae Grote, 1895. Catalog: Oregon State Arthropod Collection 3(2). (beta version). http://dx.doi.org/10.5399/osu/cat_osac.3.2.4594 Introduction These records were generated using funds from the LepNet project (Seltmann) - a national effort to create digital records for North American Lepidoptera. The dataset published herein contains the label data for all North American specimens of Lycaenidae and Riodinidae residing at the Oregon State Arthropod Collection as of March 2019. A beta version of these data records will be made available on the OSAC server (http://osac.oregonstate.edu/IPT) at the time of this publication. The beta version will be replaced in the near future with an official release (version 1.0), which will be archived as a supplemental file to this paper. Methods Basic digitization protocols and metadata standards can be found in (Shepard et al. 2018). Identifications were confirmed by Jon Shepard and Paul Hammond prior to digitization. Nomenclature follows that of (Pelham 2008). Results The holdings in these two families are extensive. Combined, they make up 25,743 specimens (24,598 Lycanidae and 1145 Riodinidae).