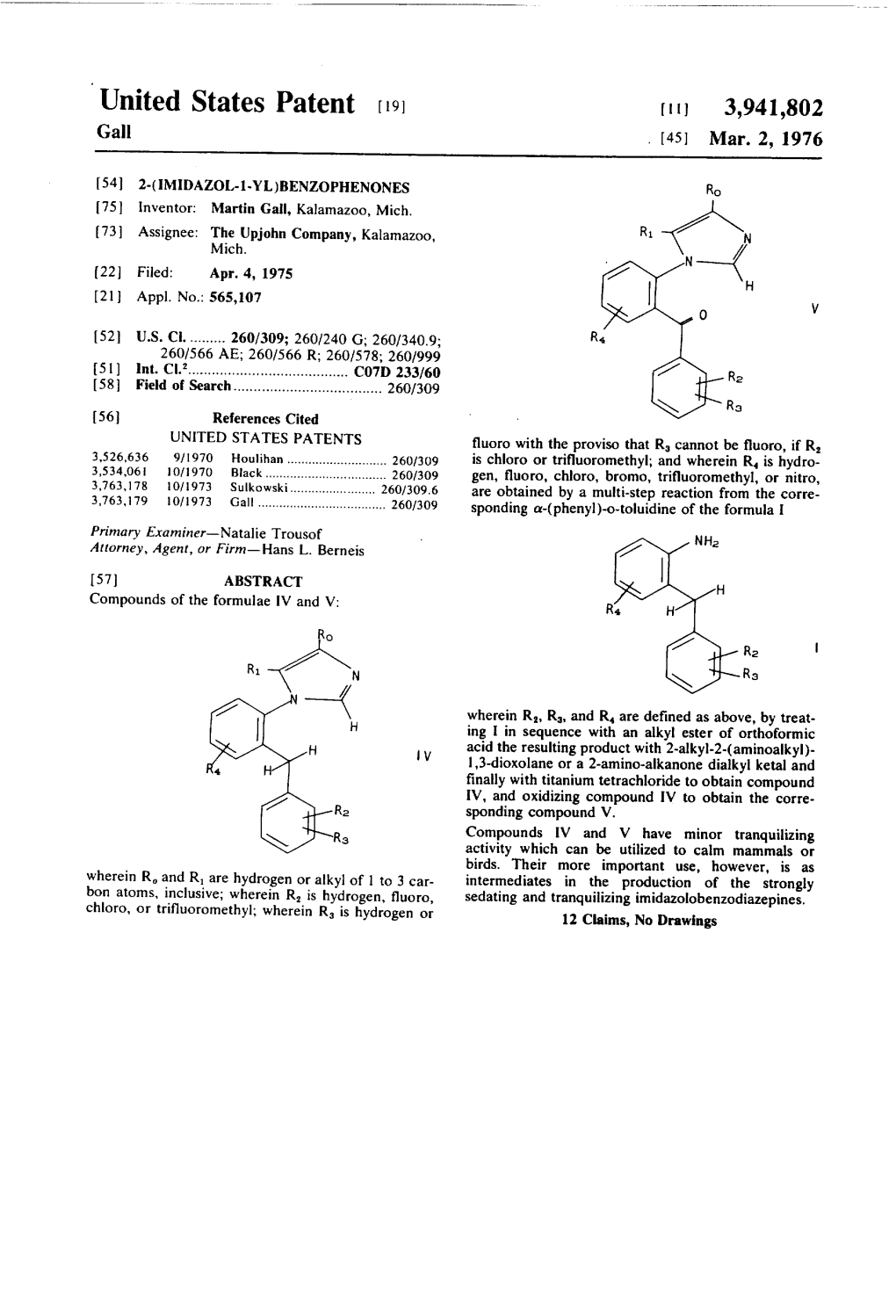
United States Patent (19) 3,941,802 Gall
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(PAC) Rev 24 Based on Applicable Aegls, Erpgs, Or Teels (Chemicals Listed by CASRN) PAC Rev 24 – August 2008
Table 3: Protective Action Criteria (PAC) Rev 24 based on applicable AEGLs, ERPGs, or TEELs (Chemicals listed By CASRN) PAC Rev 24 – August 2008 Table 3 presents a listing of chemicals and PAC data based on the Chemical Abstract Service Registry Numbers (CASRNs)1 of the chemicals. Chemicals without an identified CASRN number are issued an identification number, preceded by the letter “z,” for purposes of the PAC data set. The columns presented in Table 3 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this listing by CASRN. Chemical Name The common name of the chemical. CASRN The Chemical Abstract Service Registry Number for this chemical. TEEL-0 This is the threshold concentration below which most people will experience no appreciable risk of health effects. This PAC is always based on TEEL-0 because AEGL-0 or ERPG-0 values do not exist. PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value. PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value. PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value. Units The units for the PAC values (ppm or mg/m3). Additional information on the chemicals presented here is provided in PAC Tables 1, 2, and 4. Table 3, other PAC Tables, introductory/explanatory material (including a glossary of acronyms and abbreviations), definitions of PAC values, and alternative methods of displaying PAC information are available electronically at: http://www.hss.energy.gov/HealthSafety/WSHP/chem_safety/teel.html. -

Hazardous Substances (Chemicals) Transfer Notice 2006
16551655 OF THURSDAY, 22 JUNE 2006 WELLINGTON: WEDNESDAY, 28 JUNE 2006 — ISSUE NO. 72 ENVIRONMENTAL RISK MANAGEMENT AUTHORITY HAZARDOUS SUBSTANCES (CHEMICALS) TRANSFER NOTICE 2006 PURSUANT TO THE HAZARDOUS SUBSTANCES AND NEW ORGANISMS ACT 1996 1656 NEW ZEALAND GAZETTE, No. 72 28 JUNE 2006 Hazardous Substances and New Organisms Act 1996 Hazardous Substances (Chemicals) Transfer Notice 2006 Pursuant to section 160A of the Hazardous Substances and New Organisms Act 1996 (in this notice referred to as the Act), the Environmental Risk Management Authority gives the following notice. Contents 1 Title 2 Commencement 3 Interpretation 4 Deemed assessment and approval 5 Deemed hazard classification 6 Application of controls and changes to controls 7 Other obligations and restrictions 8 Exposure limits Schedule 1 List of substances to be transferred Schedule 2 Changes to controls Schedule 3 New controls Schedule 4 Transitional controls ______________________________ 1 Title This notice is the Hazardous Substances (Chemicals) Transfer Notice 2006. 2 Commencement This notice comes into force on 1 July 2006. 3 Interpretation In this notice, unless the context otherwise requires,— (a) words and phrases have the meanings given to them in the Act and in regulations made under the Act; and (b) the following words and phrases have the following meanings: 28 JUNE 2006 NEW ZEALAND GAZETTE, No. 72 1657 manufacture has the meaning given to it in the Act, and for the avoidance of doubt includes formulation of other hazardous substances pesticide includes but -

OZO-L-CYCLOPBNTBNE. 1-CAEBOXYLATE and ETHYL 2-METHYL-3-OXOCYCLOPENTANECAKBOXYLATE
PART I THE SYNTHESES AND EEACTIONS OF METHYL 2-METHYL-^-OZO-l-CYCLOPBNTBNE. 1-CAEBOXYLATE AND ETHYL 2-METHYL-3-OXOCYCLOPENTANECAKBOXYLATE PAET II A STUDY OF THE REACTIONS OF GRIGNARD REAGENTS WITH ESTERS OF LEVULINIC ACID DISSERTATION Presented. In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By James Li McPherson, B.S., M.A, The Ohio State University 1955 Approved by: Adviser ACKNOWLEnXSMEWT It is a pleasure to express ray deep appreciation to Dr. Melvin S. Nevraan who suggested this problem, and whose advice and encouragement were an invaluable aid throughout this investigation, and to extend to the Upjohn Company, Kalamazoo, Michigan, ray sincere appreciation for a fellowship in connection with this work. il TABLE OF CONTENTS Aoknowledgeinent Part I The Syntheses and Beaotlons of Methyl S-raethyl-l-oxo-l-cyclopentene- 1-carhoxylate (IX) and ethyl 2-methyl~5-oxocyclopentanecarboxylate (Vl) Chapter Page I Introduction 1 II Synthesis 1. Discussion of Results 12 2. Flor Sheet (Fig. 5) 15 5. Diethyl 2-cyanc«3-taethylhutanedioate (ll) l6 k . Diethyl 2-cyano-2.-(2-oyanoethyl)-3- methylhutanedioate (ill) l8 5, Tfiethyl ^-metbyl-l, $,4-pentanetricarhoxylate (l?) 19 6 , Diethyl 2-methyl-3"Oxo«l,it— cyclopentane dicarhoxylate (v) 22 7, Ethyl 2~methyl-3-oxocyclopentanecar'boxylate (Vl) 2 k 8 , 2«Methyl*-3-'Oxo"l-cyclopentene-*l« carhoxylic acid (VIIl) 26 9* 2r^ethyl-3»-oxocyclopentaneoarh0xylic acid 29 10 0 Methyl 2p-methyl"3~oxo«l-cyclopentene" 1-carhoxylate (ix) 52 11. Ethyl 2-methyl~3'*oxor«l-cyclopentene" Imcarhoxylate (VIl) 55 III Reactions 1. -

Ep 3043784 B9
(19) *EP003043784B9* (11) EP 3 043 784 B9 (12) CORRECTED EUROPEAN PATENT SPECIFICATION (15) Correction information: (51) Int Cl.: Corrected version no 1 (W1 B1) A61K 31/085 (2006.01) C07C 255/54 (2006.01) (2006.01) Corrections, see C07D 213/85 Claims EN 1, 8, 10 (86) International application number: (48) Corrigendum issued on: PCT/US2014/054375 20.11.2019 Bulletin 2019/47 (87) International publication number: (45) Date of publication and mention WO 2015/035223 (12.03.2015 Gazette 2015/10) of the grant of the patent: 15.05.2019 Bulletin 2019/20 (21) Application number: 14842085.4 (22) Date of filing: 05.09.2014 (54) ARYL ETHERS AND USES THEREOF ARYLETHER UND VERWENDUNGEN DAVON ARYLÉTHERS ET UTILISATIONS DE CEUX-CI (84) Designated Contracting States: • RIZZI, James P. AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Irving, TX 75063 (US) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • SCHLACHTER, Stephen, T. PL PT RO RS SE SI SK SM TR Dallas, TX 75208 (US) • WALLACE, Eli, M. (30) Priority: 09.09.2013 US 201361875674 P Richardson, TX 75080 (US) 11.04.2014 US 201461978421 P • WANG, Bin Dallas, TX 75229 (US) (43) Date of publication of application: • WEHN, Paul 20.07.2016 Bulletin 2016/29 Dallas, TX 75205 (US) • XU, Rui (60) Divisional application: Dallas, TX 75205 (US) 18185557.8 / 3 417 851 • YANG, Hanbiao 18185565.1 / 3 417 852 Coppell, TX 75019 (US) 19192594.0 (74) Representative: Mewburn Ellis LLP (73) Proprietor: Peloton Therapeutics, Inc. -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

Protecting Groups Third Edition by Philip J. Kocieński
Protecting Groups Third Edition by Philip J. Kocieński Protecting Groups Third Edition by Philip J. Kocieński Index acetal exchange 126, 136, 154, 160, 293, N-adenylated S-methyl-l-cysteine sulfox- 316, 566 imine 516 acetalisation 59–60, 64 Agelastatin A 508, 555 N,N-acetals 98, 369–371, 388 Agrocin 84 477 N,O-acetals 96–99, 173–179, 232, AI-77-B 576 561–568, 626–630 alanine 552 O,O-acetals 5–7, 11, 50–77, 101, 120–160, Albomycin 595 285–320, 342, 426–430 aldol reaction, directed 26 acetamidomethyl 372, 381, 384 alkene 516 acetate esters 3, 168, 206, 325, 334, 338 alkenes, terminal 55 acetate migration 196, 336 O-alkyl nucleophilic scission 399 80% acetic acid 595 alkylammonium formate 467 acetic acid 5–6, 139–140, 156, 175–176, alkylation 39, 87, 94, 370 190, 196, 200, 208, 216, 265, 269, 315, alkylation, asymmetric 411 318, 341, 343, 461, 529, 564–565, 582 S-alkylation 7 acetic acid in ether 599 alkyllithium reagents 137 acetic acid in refluxing 2-propanol 404 allene 70 acetic anhydride 156, 238, 298, 323, 497, Allopumiliotoxin 339A 245 559 Allosamidin 557 acetonides 202 allyl alcohol 606 a-acetoxy 92 allyl bromide 595 p-acetoxybenzyloxycarbonyl (AcOZ) group allyl carbamate 39 518 allyl chloroformate 284, 346, 423, 528 acetoxymethyl acetal 156 allyl esters 12, 37, 422, 517, 526 acetoxymethyl ether 157 allyl ethers 12–13, 39, 246, 276, 278, 422 2-(acetoxymethyl)benzamides 501 allyl isopropenyl dicarbonate 423 N-acetyl neuraminic acid 4, 177, 258, 330 p-allyl palladium 12, 418, 422, 594–595, 2-acetyldimedone 606 606–607 acetylene 490 -

Table 3: Protective Action Criteria (PAC) Rev. 29 Based on Applicable
Table 3: Protective Action Criteria (PAC) Rev. 29a based on applicable 60-minute AEGLs, ERPGs, or TEELs. The chemicals are listed by CASRN. June 2018 Table 3 presents a list of chemicals and associated PAC values in numerical order based on the CASRN1 for each chemical. Chemicals without an identified CASRN number are issued an identification number, preceded by the letter “z” for purposes of identification in the PAC data set. PACs are presented in either ppm or mg/m3. The columns presented in Table 3 provide the following information: Heading Definition No. The ordered numbering of the chemicals as they appear in this listing by CASRN Chemical Name The name of the chemical substance submitted to the PAC development team CASRN The Chemical Abstracts Service Registry Number for this chemical PAC-1 Based on the applicable AEGL-1, ERPG-1, or TEEL-1 value PAC-2 Based on the applicable AEGL-2, ERPG-2, or TEEL-2 value PAC-3 Based on the applicable AEGL-3, ERPG-3, or TEEL-3 value Units The units of the original request (ppm or mg/m3) Chemicals for which AEGLs are available have their chemical name, CASRN, and AEGL values displayed in a bolded and larger font. Chemicals for which ERPGs are available, but no AEGLs, have their chemical name, CASRN, and ERPG values displayed in a bolded font. Chemicals for which TEELs are available, but no AEGLs or ERPGs, have their chemical name, CASRN, and values displayed using a regular font. Additional information on PAC values, TEEL values, and links to other sources of information is provided on the Subcommittee on Consequence Assessment and Protective Actions (SCAPA) webpage at http://orise.orau.gov/emi/scapa/default.htm. -

Applications of Alkyl Orthoesters As Valuable Substrates in Organic Transformations, Focusing on Cite This: RSC Adv., 2020, 10,30314 Reaction Media
RSC Advances View Article Online REVIEW View Journal | View Issue Applications of alkyl orthoesters as valuable substrates in organic transformations, focusing on Cite this: RSC Adv., 2020, 10,30314 reaction media Zahra Khademi and Kobra Nikoofar * In this review we focus on applications of alkyl orthoesters as valuable and efficient substrates to perform various classes of two-component and multi-component organic reactions. The article has classified them according to two aspects, which are: (i) a focus on the reaction medium (solvent-free conditions, aqueous media, and organic solvents); and (ii) an examination of product structures. Reaction accomplishment under solvent-free conditions is an eco-friendly process with the absence of volatile toxic solvents, which puts it in Received 16th June 2020 line with green chemistry goals. Water is an interesting choice in organic transformations due to its Accepted 27th July 2020 inexpensiveness and safety. The authors hope their assessment will help chemists to attain new DOI: 10.1039/d0ra05276k approaches for utilizing alkyl orthoesters in various organic synthetic methods. The review covers the Creative Commons Attribution-NonCommercial 3.0 Unported Licence. rsc.li/rsc-advances corresponding literature up to the beginning of 2020. 1. Introduction (e) 3,3,3-Triethoxy-1-propyne (HCC(OEt)3, 8). Orthoesters may be considered to be products of the Orthoester (1,1,1-triorganyloxyalkane) (1) is the general name exhaustive alkylation of unstable orthocarboxylic acids and it is for a functional group containing three alkoxy groups attached from these that the name “orthoester” is derived. Orthoesters to one central carbon atom, with the general formula RC(OR')3. -

Novel Ring Forming Reactions for Organic Synthesis Jeffrey J
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1988 Novel ring forming reactions for organic synthesis Jeffrey J. Thurston Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Thurston, Jeffrey J., "Novel ring forming reactions for organic synthesis " (1988). Retrospective Theses and Dissertations. 8894. https://lib.dr.iastate.edu/rtd/8894 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Review Article a Review on the Synthesis of Scaffolds Directed Benzimidazole Based Biheterocyclic Molecules R D Padmaja, Barnali Maiti* and Kaushik Chanda*
Chemical Science Review and Letters ISSN 2278-6783 Review Article A review on the synthesis of scaffolds directed benzimidazole based biheterocyclic molecules R D Padmaja, Barnali Maiti* and Kaushik Chanda* Department of Chemistry, School of Advanced Sciences, VIT University, Vellore-632014, India Abstract Heterocyclic compounds are one of the common structural motifs for 80% of the marketed drugs reported in 2014. Benzimidazole and its derivatives are regarded as important heterocyclic motifs exhibiting a wide range of pharmaceutical applications. It is always necessary to focus on the synthesis of heterocyclic molecules contains benzimidazole as one of the moiety. This review focuses on the synthesis of different classes of benzimidazole based biheterocyclic molecules on solid support. In this review paper detailed synthetic steps were represented by schemes involved for the synthesis of benzimidazole based biheterocyclic molecules. The synthesis covers the scaffold directed linear, angular, and fused benzimidazole based biheterocyclic molecules by combinatorial approach and open a new avenue for the utilization of these privileged molecules for pharmaceutical applications. *Correspondence Author: Barnali Maiti, Kaushik Chanda Keywords: Benzimidazoles, Biheterocyclic scaffolds, Email: [email protected], polyethylene glycol, Ionic liquid [email protected] Introduction As reported by US retail sales in 2014, heterocyclic compounds play one of the major roles as these are the common structural motif for 80% of the marketed drugs in drug discovery research [1]. Therefore significant effort has been given for its construction by combinatorial approach both on the solid phase as well as on solution phase [2]. In general, the heterocyclic compounds developed by combinatorial approach having only one or two diversity points such as CH3, Et, Pr groups have limited bioactivity. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0179320 A1 Liu Et Al
US 20100179320A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0179320 A1 Liu et al. (43) Pub. Date: Jul. 15, 2010 (54) PREPARATION METHODS OF (52) U.S. Cl. ........................................... 544/319; 560/60 AZOXYSTROBIN AND ITS ANALOGS (57) ABSTRACT (76) Inventors: Shangzhong Liu, Beijing (CN); Preparation method of a compound of general formula (I) Canxian Mu, Beijing (CN); comprises the following steps: (1) a compound of general Wenjun Wang, Beijing (CN); formula (II) reacts with a formylating agent in an aprotic Jianwei Chen, Beijing (CN); solvent at a temperature between -20° C. and 200° C. in the Shuguang Wang, Beijing (CN) presence of a Lewis acid, then an organic base is added to Correspondence Address: promote the reaction to obtain an intermediate product; (2) FROMMER LAWRENCE & HAUG the above intermediate product reacts with a methylating 745 FIFTHAVENUE- 1 OTH FL. agent in the presence of an alkali at a temperature between NEW YORK, NY 10151 (US) -20°C. and 100° C. to obtain the compound of formula (I). (21) Appl. No.: 12/668,219 (I) (22) PCT Filed: Sep. 19, 2008 (86). PCT No.: PCT/CN08/72429 S371 (c)(1), (2), (4) Date: Jan. 8, 2010 (30) Foreign Application Priority Data Oct. 24, 2007 (CN) ......................... 200710163386.8 (II) Publication Classification (51) Int. Cl. C07D 239/52 (2006.01) CO7D 2.39/34 (2006.01) CD7C 67/343 (2006.01) US 2010/0179320 A1 Jul. 15, 2010 PREPARATION METHODS OF it involves additional processes including protection of start AZOXYSTROBIN AND ITS ANALOGS ing material, de-protection of intermediate product, and a series of conversions. -

(12) United States Patent (10) Patent No.: US 8,278.445 B2 Liu Et Al
USOO8278445B2 (12) United States Patent (10) Patent No.: US 8,278.445 B2 Liu et al. (45) Date of Patent: Oct. 2, 2012 (54) PREPARATION METHODS OF 7,084.272 B2 8/2006 Jackson et al. AZOXYSTROBIN AND ITS ANALOGS 2004/0242607 A1 12/2004 Jackson et al. 2006/022.9450 A1 10, 2006 Jackson et al. (75) Inventors: Shangzhong Liu, Beijing (CN); 2006/0287527 A1 12/2006 Miyazawa et al. Canxian Mu, Beijing (CN); Wenjun FOREIGN PATENT DOCUMENTS Wang, Beijing (CN); Jianwei Chen, CN 1016611 2, 1988 Beijing (CN); Shuguang Wang, Beijing CN 12064O1 1, 1999 (CN) CN 1511144 T 2004 CN 101.157657 4/2008 (73) Assignee: Nutrichem Company Limited, Beijing (CN) Primary Examiner — James O Wilson Assistant Examiner — Ebenezer O Sackey (*) Notice: Subject to any disclaimer, the term of this (74) Attorney, Agent, or Firm — Orrick, Herrington & patent is extended or adjusted under 35 Sutcliffe, LLP U.S.C. 154(b) by 291 days. (21) Appl. No.: 12/668,219 (57) ABSTRACT Preparation method of a compound of general formula (I) (22) PCT Filed: Sep.19, 2008 comprises the following steps: (1) a compound of general formula (II) reacts with a formylating agent in an aprotic (86). PCT No.: PCT/CN2008/072429 solvent at a temperature between -20° C. and 200° C. in the S371 (c)(1), presence of a Lewis acid, then an organic base is added to promote the reaction to obtain an intermediate product; (2) (2), (4) Date: Jan. 8, 2010 the above intermediate product reacts with a methylating (87) PCT Pub. No.: WO2009/052719 agent in the presence of an alkali at a temperature between -20°C.