Table 3: Protective Action Criteria (PAC) Rev. 29 Based on Applicable
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Quality Testing Summary
WATER QUALITY TESTING SUMMARY A DETAILED REVIEW OF THE TEST RESULTS FOR THE DRINKING WATER PRODUCED BY THE CARY/APEX WATER TREATMENT FACILITY 2020 JOHN CONLEY (Senior Laboratory Analyst) has been employed by the Town of Cary at the Cary/Apex Water Treatment Facility Laboratory since September 2001. CARY/APEX WATER TREATMENT FACILITY 2020 WATER QUALITY TESTING SUMMARY We are pleased to present to you the Cary/ If you have any questions or concerns regarding this Apex Water Treatment Facility Test Result report, please contact Rachel Monschein, Water System Summary for 2020. This report is a snapshot of last Laboratory Supervisor, at (919) 362-5507. year’s water quality. The values contained in this report In order to ensure that tap water is safe to drink, EPA are based on single measurements or yearly averages depending on the contaminant. The Environmental prescribes regulations that limit the amount of certain Protection Agency and/or the State requires us to contaminants in water provided by public water systems. monitor for certain substances less than once per year Drinking water, including bottled water, may reasonably because the concentrations of these substances are not be expected to contain at least small amounts of some expected to vary significantly from year to year. Some of contaminants. The presence of contaminants does not the data, though representative of the water quality, is necessarily indicate the water poses a health risk. To obtain more than one year old. In these cases, the most recent more information about contaminants and potential data is included, along with the year in which the sample health effects, call the EPA’s Safe Drinking Water Hotline was taken. -

An Aged Reaction Revisited
TETRAHEDRON Pergamon Tetrahedron 56 (2000) 9705±9711 2-Alkyl-4,6-dialkylamino-1,3,5-triazines via Grignard Alkylation of Cyanuric Chloride: An Aged Reaction Revisited Rita Menicagli,* Simona Samaritani and Valeria Zucchelli Dipartimento di Chimica e Chimica Industriale and Centro di Studi del CNR per le Macromolecole Stereordinate ed Otticamente Attive, Via Risorgimento 35, 56126 Pisa, Italy Received 24 July 2000; revised 8 September 2000; accepted 28 September 2000 AbstractÐSuitable one-pot reaction conditions are suggested to prepare, in good overall yields, some 2-(alk-1 0-ynyl)- and 2-alkyl-4,6- dialkylamino-1,3,5-triazines via reaction of cyanuric chloride with Grignard reagents followed by amination. q 2000 Elsevier Science Ltd. All rights reserved. In our studies1 concerning the protection of paper against The reaction between a benzene (CAUTION) solution of 1 pathogenic fungi, we found that 2-(alk-1 0-ynyl)-4,6- and a THF solution of alk-1 0-ynylmagnesium halides has dimethoxy-1,3,5-triazines showed an appreciable biostatic been reported to afford 2-(alk-1 0-ynyl)-4,6-dichloro-1,3,5- activity.2 Taking into account that alkylamino derivatives of triazines in 50±60% yield.6a Since the puri®cation of these 1,3,5-triazine are generally better biostatic agents than intermediates might have caused an appreciable loss of the alkoxy derivatives,1 preparation of 2-(alk-1 0-ynyl)-4,6- product owing to the well known8 reactivity of the C±Cl dialkylamino-1,3,5-triazines was necessary in order to bonds and the treatment of the crude products with nucleo- compare their antifungal activities with those of 2-(alk-1 0- philes would have lead to complex mixtures of compounds, ynyl)-4,6-dimethoxy-1,3,5-triazines. -

Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446
Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION MEMORANDUM Date: March 11, 2020 SUBJECT: Registration Review Draft Risk Assessment for the Peroxy Compounds PC Code: 000595, 063201, 063604, 063607, DP Barcode: 455445, 455446 063209, 128860 Decision No: 558073, 558074 Docket No: EPA-HQ-OPP-2009-0546 Regulatory Action: Registration Review Case No: 6059, 4072, 5081 Risk Assessment Type: DRA CAS No: 7722-84-1, 79-21-0, 33734-57-5, 15630-89-4, 10058-23-8, 70693-62-8 TO: Kendall Ziner, Chemical Review Manager Rick Fehir, Ph.D., Team Lead Rose Kyprianou, Branch Chief Regulatory Management Branch (RMB) II Antimicrobials Division (7510P) Office of Pesticide Programs FROM: Andrew Byro, Ph.D., Chemist Kathryn Korthauer, Biologist Timothy Dole, Industrial Hygienist Deborah Burgin, Ph.D., DABT, Toxicologist Risk Assessment and Science Support Branch Antimicrobials Division (7510P) Office of Pesticide Programs THROUGH: Judy Facey, Ph.D., Human Health Risk Assessment Process Leader MP for JF Diana Hsieh, Ecological Risk Assessment Process Leader MP for DH Timothy Leighton, Senior Science Advisor MP for TL Laura Parsons, Associate Branch Chief Melissa Panger, Ph.D., Branch Chief Risk Assessment and Science Support Branch Antimicrobials Division (7510P) This document provides the draft human health and ecological risk assessment conducted in support of the antimicrobial use sites of the following peroxy compounds: hydrogen peroxide, peracetic acid, peroxyoctanoic acid, and sodium percarbonate. Page 1 of 74 Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 Although the peroxymonosulfate compounds were included in the peroxy compounds Final Work Plan (FWP), they will not be included in this risk assessment. -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

WO 2015/179628 Al 26 November 2015 (26.11.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/179628 Al 26 November 2015 (26.11.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C08F 210/16 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/US20 15/03 1952 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 2 1 May 2015 (21 .05.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 14/284,689 22 May 2014 (22.05.2014) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant: CHEVRON PHILLIPS CHEMICAL COM¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, PANY LP [US/US]; 10001 Six Pines Drive, The Wood TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, lands, TX 77380 (US). -

01 Excipients Prelims 1..9
Ethylparaben 1 Nonproprietary Names BP: Ethyl Hydroxybenzoate SEM 1: Excipient: ethylparaben; magnification: 600Â. JP: Ethyl Parahydroxybenzoate PhEur: Ethyl Parahydroxybenzoate E USP-NF: Ethylparaben 2 Synonyms Aethylum hydrobenzoicum; CoSept E; E214; ethylis parahydroxy- benzoas; ethyl p-hydroxybenzoate; Ethyl parasept; 4-hydroxyben- zoic acid ethyl ester; Nipagin A; Solbrol A; Tegosept E; Uniphen P- 23. 3 Chemical Name and CAS Registry Number Ethyl-4-hydroxybenzoate [120-47-8] 4 Empirical Formula and Molecular Weight C9H10O3 166.18 5 Structural Formula SEM 2: Excipient: ethylparaben; magnification: 3000Â. 6 Functional Category Antimicrobial preservative. 7 Applications in Pharmaceutical Formulation or Technology Ethylparaben is widely used as an antimicrobial preservative in cosmetics,(1) food products, and pharmaceutical formulations. It may be used either alone or in combination with other paraben esters or with other antimicrobial agents. In cosmetics it is one of the most frequently used preservatives. The parabens are effective over a wide pH range and have a broad spectrum of antimicrobial activity, although they are most effective against yeasts and molds; see Section 10. Owing to the poor solubility of the parabens, paraben salts, particularly the sodium salt, are frequently used. However, this may cause the pH of poorly buffered formulations to become more alkaline. See Methylparaben for further information. 8 Description Ethylparaben occurs as a white, odorless or almost odorless, active against yeasts and molds than against bacteria. They crystalline powder. are also more active against Gram-positive than against Gram-negative bacteria. 9 Pharmacopeial Specifications The activity of the parabens increases with increasing chain See Table I. See also Section 18. length of the alkyl moiety, but solubility decreases. -

Calarp) Program
California Accidental Release Prevention (CalARP) Program Administering Agency Guidance January 31, 2005 Preface This document provides general guidance to help Administering Agencies (AAs) implement and enforce the California Accidental Release Prevention (CalARP) Program. The intent is to identify the elements of the Program applicable to each regulated business, and assist AAs with oversight of the CalARP Program statutes and regulations. This document is not a substitute for the CalARP Program regulations; it does not impose legally binding requirements. About This Document This document follows the format of the California Code of Regulations, Title 19, Division 2, Chapter 4.5: California Accidental Release Prevention (CalARP) Program. The regulatory sections are presented in parentheses for ease of reference. Acknowledgements The California Emergency Management Agency (Cal EMA) would like to thank the following people for their valuable assistance in the preparation of this document: Howard Wines, Hazardous Materials Specialist, City of Bakersfield Fire Department Robert Distaso P.E., Fire Safety Engineer, Orange County Fire Authority Randall L. Sawyer, Supervisor, Accidental Release Prevention Programs, Contra Costa County Health Services Department Beronia Beniamine, Senior Hazardous Materials Specialist, Stanislaus County Environmental Resources Department Angie Proboszcz, Risk Management Program Coordinator, USEPA Region 9 Jon Christenson, Senior Environmental Health Specialist, Merced County Department of Public Health Teresa -

Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects
EN58CH06-Casida ARI 5 December 2012 8:11 Neuroactive Insecticides: Targets, Selectivity, Resistance, and Secondary Effects John E. Casida1,∗ and Kathleen A. Durkin2 1Environmental Chemistry and Toxicology Laboratory, Department of Environmental Science, Policy, and Management, 2Molecular Graphics and Computational Facility, College of Chemistry, University of California, Berkeley, California 94720; email: [email protected], [email protected] Annu. Rev. Entomol. 2013. 58:99–117 Keywords The Annual Review of Entomology is online at acetylcholinesterase, calcium channels, GABAA receptor, nicotinic ento.annualreviews.org receptor, secondary targets, sodium channel This article’s doi: 10.1146/annurev-ento-120811-153645 Abstract Copyright c 2013 by Annual Reviews. Neuroactive insecticides are the principal means of protecting crops, people, All rights reserved livestock, and pets from pest insect attack and disease transmission. Cur- ∗ Corresponding author rently, the four major nerve targets are acetylcholinesterase for organophos- phates and methylcarbamates, the nicotinic acetylcholine receptor for neonicotinoids, the γ-aminobutyric acid receptor/chloride channel for by Public Health Information Access Project on 04/29/14. For personal use only. Annu. Rev. Entomol. 2013.58:99-117. Downloaded from www.annualreviews.org polychlorocyclohexanes and fiproles, and the voltage-gated sodium channel for pyrethroids and dichlorodiphenyltrichloroethane. Species selectivity and acquired resistance are attributable in part to structural differences in binding subsites, receptor subunit interfaces, or transmembrane regions. Additional targets are sites in the sodium channel (indoxacarb and metaflumizone), the glutamate-gated chloride channel (avermectins), the octopamine receptor (amitraz metabolite), and the calcium-activated calcium channel (diamides). Secondary toxic effects in mammals from off-target serine hydrolase inhibi- tion include organophosphate-induced delayed neuropathy and disruption of the cannabinoid system. -

Appendix H EPA Hazardous Waste Law
Appendix H EPA Hazardous Waste Law This Appendix is intended to give you background information on hazardous waste laws and how they apply to you. For most U.S. Environmental Protection Agency (EPA) requirements that apply to the University, the Safety Department maintains compliance through internal inspections, record keeping and proper disposal. In Wisconsin, the Department of Natural Resources (DNR) has adopted the EPA regulations, consequently EPA and DNR regulations are nearly identical. EPA defines This Appendix only deals with "hazardous waste" as defined by the EPA. hazardous waste as Legally, EPA defines hazardous waste as certain hazardous chemical waste. This hazardous chemical Appendix does not address other types of regulated laboratory wastes, such as waste; radioactive, infectious, biological, radioactive or sharps. Chapter 8 descibes disposal procedures infectious and biohazardous waste for animals. Chapter 9 describes disposal procedures for sharps and other waste that are regulated by can puncture tissue. Chapter 11 discusses Radiation and the Radiation Safety for other agencies. Radiation Workers provides guidelines for the disposal of radioactive waste. Procedures for medical waste are written by the UW Hospital Safety Officer. The Office of Biological Safety can provide guidance for the disposal of infectious and biological waste. EPA regulations focus on industrial waste streams. As a result, many laboratory chemical wastes are not regulated by EPA as hazardous chemical waste. However, many unregulated chemical wastes do merit special handling and disposal If a waste can be procedures. Thus, Chapter 7 and Appendix A of this Guide recommend disposal defined as: procedures for many unregulated wastes as if they were EPA hazardous waste. -
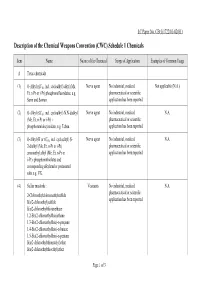
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention. -

CF 7.4 V2.Indd
2007 VOLUME 7 NUMBER 4 Product Directory Grignard and Organozinc Reagents RIEKE® HIGHLY REACTIVE METALS GRIGNARD REAGENTS ORGANOZINC HALIDES DIALKYLMAGNESIUM AND DIALKYZINC REAGENTS 2-Pyridylzinc bromide: a shelf-stable 2-pyridyl anion equivalent; an important motif in many pharmacologically active molecules. sigma-aldrich.com 2 Table of Contents Sigma-Aldrich is committed to providing the most extensive portfolio of high-quality Grignard, organozinc, and other organometallic reagents, and we continually expand our product listing. Within each section of this directory, products are listed by increasing carbon content. Rieke® Highly Reactive Metals If viewing the electronic version simply select Grignard Reagents a category to jump to that section or activate Alkyl Alkenyl Alkynyl Aryl Heteroaryl your Adobe Bookmarks. You may also search by name, product number, molecular formula, Organozinc Halides or CAS registry number simply by using the “find” feature in Adobe (Ctrl+F in Windows or Alkyl Alkenyl Aryl Heteroaryl Introduction Command+F in a Mac environment). Dialkylmagnesium and Dialkylzinc Reagents If you are unable to find a reagent for your research “Please Bother Us” at [email protected], or contact your local Sigma-Aldrich office (see back cover). Foreword Reuben D. Rieke President and CEO, Rieke Metals, Inc. Professor Emeritus, University of Nebraska Lincoln, NE In the last 35 years, considerable research has been done in the area of generating reactive metals that can be used to synthesize novel organometallic reagents. In 1972, we reported a general approach for preparing highly reactive metal powders, relying on the reduction of metal salts with alkali metals in ethereal or hydrocarbon solvents.