TEMPERATURE EFFECTS on the ACTIVITY COEFFICIENT of the BICARBONATE ION Thesis by Fernando Cadena Cepeda in Partial Fulfillment O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prediction of Osmotic Coefficients by Pair Correlation Function Method" (1991)
New Jersey Institute of Technology Digital Commons @ NJIT Dissertations Electronic Theses and Dissertations Spring 1-31-1991 Thermodynamics of strong electrolyte solutions : prediction of osmotic coefficientsy b pair correlation function method One Kwon Rim New Jersey Institute of Technology Follow this and additional works at: https://digitalcommons.njit.edu/dissertations Part of the Chemical Engineering Commons Recommended Citation Rim, One Kwon, "Thermodynamics of strong electrolyte solutions : prediction of osmotic coefficients by pair correlation function method" (1991). Dissertations. 1145. https://digitalcommons.njit.edu/dissertations/1145 This Dissertation is brought to you for free and open access by the Electronic Theses and Dissertations at Digital Commons @ NJIT. It has been accepted for inclusion in Dissertations by an authorized administrator of Digital Commons @ NJIT. For more information, please contact [email protected]. Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be -

Thermodynamic Properties of 1:1 Salt Aqueous Solutions with the Electrolattice M
D o s s i e r This paper is a part of the hereunder thematic dossier published in OGST Journal, Vol. 68, No. 2, pp. 187-396 and available online here Cet article fait partie du dossier thématique ci-dessous publié dans la revue OGST, Vol. 68, n°2, pp. 187-396 et téléchargeable ici © Photos: IFPEN, Fotolia, X. DOI: 10.2516/ogst/2012094 DOSSIER Edited by/Sous la direction de : Jean-Charles de Hemptinne InMoTher 2012: Industrial Use of Molecular Thermodynamics InMoTher 2012 : Application industrielle de la thermodynamique moléculaire Oil & Gas Science and Technology – Rev. IFP Energies nouvelles, Vol. 68 (2013), No. 2, pp. 187-396 Copyright © 2013, IFP Energies nouvelles 187 > Editorial électrostatique ab initio X. Rozanska, P. Ungerer, B. Leblanc and M. Yiannourakou 217 > Improving the Modeling of Hydrogen Solubility in Heavy Oil Cuts Using an Augmented Grayson Streed (AGS) Approach 309 > Improving Molecular Simulation Models of Adsorption in Porous Materials: Modélisation améliorée de la solubilité de l’hydrogène dans des Interdependence between Domains coupes lourdes par l’approche de Grayson Streed Augmenté (GSA) Amélioration des modèles d’adsorption dans les milieux poreux R. Torres, J.-C. de Hemptinne and I. Machin par simulation moléculaire : interdépendance entre les domaines J. Puibasset 235 > Improving Group Contribution Methods by Distance Weighting Amélioration de la méthode de contribution du groupe en pondérant 319 > Performance Analysis of Compositional and Modified Black-Oil Models la distance du groupe For a Gas Lift Process A. Zaitseva and V. Alopaeus Analyse des performances de modèles black-oil pour le procédé d’extraction par injection de gaz 249 > Numerical Investigation of an Absorption-Diffusion Cooling Machine Using M. -

Dissociation Constants and Ph-Titration Curves at Constant Ionic Strength from Electrometric Titrations in Cells Without Liquid
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1537 Part of Journal of Research of the N.ational Bureau of Standards, Volume 30, May 1943 DISSOCIATION CONSTANTS AND pH-TITRATION CURVES AT CONSTANT IONIC STRENGTH FROM ELECTRO METRIC TITRATIONS IN CELLS WITHOUT LIQUID JUNCTION : TITRATIONS OF FORMIC ACID AND ACETIC ACID By Roger G. Bates, Gerda L. Siegel, and S. F. Acree ABSTRACT An improved method for obtaining the titration curves of monobasic acids is outlined. The sample, 0.005 mole of the sodium salt of the weak acid, is dissolver! in 100 ml of a 0.05-m solution of sodium chloride and titrated electrometrically with an acid-salt mixture in a hydrogen-silver-chloride cell without liquid junction. The acid-salt mixture has the composition: nitric acid, 0.1 m; pot assium nitrate, 0.05 m; sodium chloride, 0.05 m. The titration therefore is performed in a. medium of constant chloride concentration and of practically unchanging ionic strength (1'=0.1) . The calculations of pH values and of dissociation constants from the emf values are outlined. The tit ration curves and dissociation constants of formic acid and of acetic acid at 25 0 C were obtained by this method. The pK values (negative logarithms of the dissociation constants) were found to be 3.742 and 4. 754, respectively. CONTENTS Page I . Tntroduction __ _____ ~ __ _______ . ______ __ ______ ____ ________________ 347 II. Discussion of the titrat ion metbod __ __ ___ ______ _______ ______ ______ _ 348 1. Ti t;at~on. clU,:es at constant ionic strength from cells without ltqUld JunctlOlL - - - _ - __ _ - __ __ ____ ____ _____ __ _____ ____ __ _ 349 2. -

Simple Mixtures
Simple mixtures Before dealing with chemical reactions, here we consider mixtures of substances that do not react together. At this stage we deal mainly with binary mixtures (mixtures of two components, A and B). We therefore often be able to simplify equations using the relation xA + xB = 1. A restriction in this chapter – we consider mainly non-electrolyte solutions – the solute is not present as ions. The thermodynamic description of mixtures We already considered the partial pressure – the contribution of one component to the total pressure, when we were dealing with the properties of gas mixtures. Here we introduce other analogous partial properties. Partial molar quantities: describe the contribution (per mole) that a substance makes to an overall property of mixture. The partial molar volume, VJ – the contribution J makes to the total volume of a mixture. Although 1 mol of a substance has a characteristic volume when it is pure, 1 mol of a substance can make different contributions to the total volume of a mixture because molecules pack together in different ways in the pure substances and in mixtures. Imagine a huge volume of pure water. When a further 1 3 mol H2O is added, the volume increases by 18 cm . When we add 1 mol H2O to a huge volume of pure ethanol, the volume increases by only 14 cm3. 18 cm3 – the volume occupied per mole of water molecules in pure water. 14 cm3 – the volume occupied per mole of water molecules in virtually pure ethanol. The partial molar volume of water in pure water is 18 cm3 and the partial molar volume of water in pure ethanol is 14 cm3 – there is so much ethanol present that each H2O molecule is surrounded by ethanol molecules and the packing of the molecules results in the water molecules occupying only 14 cm3. -

Chapter 9 Ideal and Real Solutions
2/26/2016 CHAPTER 9 IDEAL AND REAL SOLUTIONS • Raoult’s law: ideal solution • Henry’s law: real solution • Activity: correlation with chemical potential and chemical equilibrium Ideal Solution • Raoult’s law: The partial pressure (Pi) of each component in a solution is directly proportional to the vapor pressure of the corresponding pure substance (Pi*) and that the proportionality constant is the mole fraction (xi) of the component in the liquid • Ideal solution • any liquid that obeys Raoult’s law • In a binary liquid, A-A, A-B, and B-B interactions are equally strong 1 2/26/2016 Chemical Potential of a Component in the Gas and Solution Phases • If the liquid and vapor phases of a solution are in equilibrium • For a pure liquid, Ideal Solution • ∆ ∑ Similar to ideal gas mixing • ∆ ∑ 2 2/26/2016 Example 9.2 • An ideal solution is made from 5 mole of benzene and 3.25 mole of toluene. (a) Calculate Gmixing and Smixing at 298 K and 1 bar. (b) Is mixing a spontaneous process? ∆ ∆ Ideal Solution Model for Binary Solutions • Both components obey Rault’s law • Mole fractions in the vapor phase (yi) Benzene + DCE 3 2/26/2016 Ideal Solution Mole fraction in the vapor phase Variation of Total Pressure with x and y 4 2/26/2016 Average Composition (z) • , , , , ,, • In the liquid phase, • In the vapor phase, za x b yb x c yc Phase Rule • In a binary solution, F = C – p + 2 = 4 – p, as C = 2 5 2/26/2016 Example 9.3 • An ideal solution of 5 mole of benzene and 3.25 mole of toluene is placed in a piston and cylinder assembly. -

CH. 9 Electrolyte Solutions
CH. 9 Electrolyte Solutions 9.1 Activity Coefficient of a Nonvolatile Solute in Solution and Osmotic Coefficient for the Solvent As shown in Ch. 6, the chemical potential is 0 where i is the chemical potential in the standard state and is a measure of concentration. For a nonvolatile solute, its pure liquid is often not a convenient standard state because a pure nonvolatile solute cannot exist as a liquid. For the dissolved solute, * i is the chemical potential of i in a hypothetical ideal solution at unit concentration (i = 1). In the ideal solution i = 1 for all compositions In real solution, i 1 as i 0 A common misconception: The standard state for the solute is the solute at infinite dilution. () At infinite dilution, the chemical potential of the solute approaches . Thus, the standard state should be at some non-zero concentration. A standard state need not be physically realizable, but it must be well- defined. For convenience, unit concentration i = 1 is used as the standard state. Three composition scales: Molarity (moles of solute per liter of solution, ci) The standard state of the solute is a hypothetical ideal 1-molar solution of i. (c) In real solution, i 1 as ci 0 Molality (moles of solute per kg of solvent, mi) // commonly used for electrolytes // density of solution not needed The standard state is hypothetical ideal 1-molal solution of i. (m) In real solution, i 1 as mi 0 Mole fraction xi Molality is an inconvenient scale for concentrated solution, and the mole fraction is a more convenient scale. -
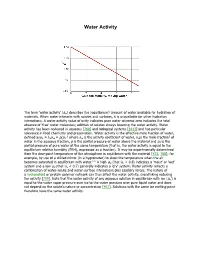
Water Activity
Water Activity i The term 'water activity' (aw) describes the (equilibrium) amount of water available for hydration of materials. When water interacts with solutes and surfaces, it is unavailable for other hydration interactions. A water activity value of unity indicates pure water whereas zero indicates the total absence of 'free' water molecules; addition of solutes always lowering the water activity. Water activity has been reviewed in aqueous [788] and biological systems [1813] and has particular relevance in food chemistry and preservation. Water activity is the effective mole fraction of water, a g defined asaw = λwxw = p/p0 where λw is the activity coefficient of water, xwis the mole fraction of water in the aqueous fraction, p is the partial pressure of water above the material and p0 is the partial pressure of pure water at the same temperature (that is, the water activity is equal to the equilibrium relative humidity (ERH), expressed as a fraction). It may be experimentally determined from the dew-point temperature of the atmosphere in equilibrium with the material [473, 788]; for example, by use of a chilled mirror (in a hygrometer) to show the temperature when the air f, h becomes saturated in equilibrium with water. A high aw (that is, > 0.8) indicates a 'moist' or 'wet' system and a low aw (that is, < 0.7) generally indicates a 'dry' system. Water activity reflects a combination of water-solute and water-surface interactions plus capillary forces. The nature of a hydrocolloid or protein polymer network can thus affect the water activity, crosslinking reducing i the activity [759]. -

Friedman's Excess Free Energy and the Mcmillan–Mayer Theory Of
Pure Appl. Chem., Vol. 85, No. 1, pp. 105–113, 2013. http://dx.doi.org/10.1351/PAC-CON-12-05-08 © 2012 IUPAC, Publication date (Web): 29 December 2012 Friedman’s excess free energy and the McMillan–Mayer theory of solutions: Thermodynamics* Juan Luis Gómez-Estévez Departament de Física Fonamental, Facultat de Física, Universitat de Barcelona, Diagonal 645, E-08028, Barcelona, Spain Abstract: In his version of the theory of multicomponent systems, Friedman used the anal- ogy which exists between the virial expansion for the osmotic pressure obtained from the McMillan–Mayer (MM) theory of solutions in the grand canonical ensemble and the virial expansion for the pressure of a real gas. For the calculation of the thermodynamic properties of the solution, Friedman proposed a definition for the “excess free energy” that is a reminder of the ancient idea for the “osmotic work”. However, the precise meaning to be attached to his free energy is, within other reasons, not well defined because in osmotic equilibrium the solution is not a closed system and for a given process the total amount of solvent in the solu- tion varies. In this paper, an analysis based on thermodynamics is presented in order to obtain the exact and precise definition for Friedman’s excess free energy and its use in the compar- ison with the experimental data. Keywords: excess functions; free energy; Friedman; McMillan–Mayer; osmotic pressure; theory of solutions. INTRODUCTION The McMillan–Mayer (MM) theory of solutions [1–6] is a general theory that was originally formu- lated within the context of the grand canonical ensemble. -

Section 1: Identification
RPP-RPT-50703 Rev.01A 8/23/2016 - 10:10 AM 1 of 77 Release Stamp DOCUMENT RELEASE AND CHANGE FORM Prepared For the U.S. Department of Energy, Assistant Secretary for Environmental Management By Washington River Protection Solutions, LLC., PO Box 850, Richland, WA 99352 Contractor For U.S. Department of Energy, Office of River Protection, under Contract DE-AC27-08RV14800 DATE: TRADEMARK DISCLAIMER: Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof or its contractors or subcontractors. Printed in the United States of America. Aug 23, 2016 1. Doc No: RPP-RPT-50703 Rev. 01A 2. Title: Development of a Thermodynamic Model for the Hanford Tank Waste Simulator (HTWOS) 3. Project Number: ☒ N/A 4. Design Verification Required: ☐ Yes ☒ No 5. USQ Number: ☒ N/A 6. PrHA Number Rev. ☒ N/A Clearance Review Restriction Type: public 7. Approvals Title Name Signature Date Checker CREE, LAURA H CREE, LAURA H 08/16/2016 Clearance Review RAYMER, JULIA R RAYMER, JULIA R 08/23/2016 Document Control Approval MANOR, TAMI MANOR, TAMI 08/23/2016 Originator BRITTON, MICHAEL D BRITTON, MICHAEL D 08/16/2016 Quality Assurance DELEON, SOSTEN O DELEON, SOSTEN O 08/16/2016 Responsible Manager CREE, LAURA H CREE, LAURA H 08/16/2016 8. Description of Change and Justification Updated the reduced chemical potential coefficient vlaues for Na2SO4 and Na2SO4·10H2O in Table A.1, as the original values were incorrect. -
![Activity Coefficient Varies with Ionic Strength Activity of an Ion Ai = [Xi] ϒi](https://docslib.b-cdn.net/cover/2385/activity-coefficient-varies-with-ionic-strength-activity-of-an-ion-ai-xi-i-3232385.webp)
Activity Coefficient Varies with Ionic Strength Activity of an Ion Ai = [Xi] ϒi
Chapter 9: Effects of Electrolytes on Chemical Equilibria: Activities Please do problems: 1,2,3,6,7,8,12 Exam II February 13 Chemistry Is Not Ideal ● The ideal gas law: PV = nRT works only under “ideal situations when when pressures are low and temperatures not too low. Under non-ideal conditons we must use the Van der Wals equation to get accurate results. ● Ideal to non-ideal formalisms are common in science ● With equilibrium constants we see the same. Our idealistic case is dilute solutions. As we move away from dilute situation our equilibrium expressions become unreliable. We deal with this using a new way to express “effective concentration” called “activity” Our Ideal Equilbrium Constants Don’t Always Work [C]c[D]d aA + bB cC + dD Kc = [A]a[B]b 3+ - 3+ - 3 Al(OH)3(s) Al (aq) + 3OH (aq) Ksp = [Al ][OH ] [H+] HCOOH (aq) H+ (aq) + HCOO- (aq) K = a [A-] [HA] + - [H3O ][OH ] H O(l) + H O(l) H O+ + OH– K = 2 2 3 w 2 [H2O] Electrolyte Concentrations Impact Kc Values Must Use Activity Molarity Works Fine Here [Electrolytes] Affect Equilibrium ● Addition of an electrolyte (as concentration increase) tends to increase solubility. ● We need a better Kc than one using molarities. We use “effective concentration” which is called activity. Activity of An Ion is “effective concentration” aA + bB cC + dD c d equilibrium [C] [D] constatnt in Kc = a b terms of molar [A] [B] concentration c d equilibrium ay az constant in Ka = a b terms of aw bx activities Activity of An Ion is “effective concentration” In order to take into the effects of electrolytes on chemical equilibria we use “activity” instead of “concentration”. -

3. Activity Coefficients of Aqueous Species 3.1. Introduction
3. Activity Coefficients of Aqueous Species 3.1. Introduction The thermodynamic activities (ai) of aqueous solute species are usually defined on the basis of molalities. Thus, they can be described by the product of their molal concentrations (mi) and their γ molal activity coefficients ( i): γ ai = mi i (77) The thermodynamic activity of the water (aw) is always defined on a mole fraction basis. Thus, it can be described analogously by product of the mole fraction of water (xw) and its mole fraction λ activity coefficient ( w): λ aw = xw w (78) It is also possible to describe the thermodynamic activities of aqueous solutes on a mole fraction (x) basis. However, such mole fraction-based activities (ai ) are not the same as the more familiar (m) molality-based activities (ai ), as they are defined with respect to different choices of standard λ states. Mole fraction based activities and activity coefficients ( i), are occasionally applied to aqueous nonelectrolyte species, such as ethanol in water. In geochemistry, the aqueous solutions of interest almost always contain electrolytes, so mole-fraction based activities and activity co- efficients of solute species are little more than theoretical curiosities. In EQ3/6, only molality- (m) based activities and activity coefficients are used for such species, so ai always implies ai . Be- cause of the nature of molality, it is not possible to define the activity and activity coefficient of (x) water on a molal basis; thus, aw always means aw . Solution thermodynamics is a construct designed to approximate reality in terms of deviations from some defined ideal behavior. -

Electrolyte Solutions: Thermodynamics, Crystallization, Separation Methods
Downloaded from orbit.dtu.dk on: Oct 06, 2021 Electrolyte Solutions: Thermodynamics, Crystallization, Separation methods Thomsen, Kaj Link to article, DOI: 10.11581/dtu:00000073 Publication date: 2009 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Thomsen, K. (2009). Electrolyte Solutions: Thermodynamics, Crystallization, Separation methods. https://doi.org/10.11581/dtu:00000073 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Electrolyte Solutions: Thermodynamics, Crystallization, Separation methods 2009 Kaj Thomsen, Associate Professor, DTU Chemical Engineering, Technical University of Denmark [email protected] 1 List of contents 1 INTRODUCTION ................................................................................................................. 5 2 CONCENTRATION