The Iris- Empress of Flowers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
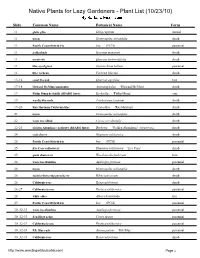
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

Philip J. Savage, Jr
ISSUE 73 M4SNOU4 Philip J. Savage, Jr. May 8, 1917 — October 13, 2002 Phil Savnge, /r. , an icon m the Magnolia worhl, passed au ay last October of conr plica- lions from the West Nile virus. Phil, a renotvrred magnolin Irybcidrzer, specialized in breeding mngnolias that u&cre cold Irardy. Becnuse of this, nragnolia entirusiasts living in colder climalcs now have morry rrrorc choices thnn did Dennis Ledvh rn u&hen he bought Iris lrouse irr Green Bay, Wiscorrsin in thc Intr typos Plril hns left an errduring legaclt n&itlr tire mnny /Inc, cold-lurrdy hybrids Ire bred. Following arc set&eral rwni niscclrces fronr irrdividuals who toere deqviy in/luencerl by Phil mrd Iris u&ork u&ith mngrrolins. DENNIs LEDVINA WIUTES. Back in the late yos the landscaping around my new house consisted of three magnolias: two M. x sorrlarrgeana, and a M. x loebneri 'MerrilL' At the time, these were the only magnolias gener- ally available at local nurseries. As I watched these magnolias bloom each spring, I became more intrigued with their beautiful Bowers and began driv- ing around Green Bay to observe and admire some of the established trees. My admiration for the genus contin- ued to grow each year as I began col- lecting more information about these magnificent plants. One summer I was in the Detroit area and I decided to call this magnolia expert, Phil Savage, that I had read so much about. I can vividly remember calling Phil from a telephone booth on Telegraph Road and finding mysell, an unknown amateur, talking to a magnolia expert who from the first made me feel like a lifelong friend. -

May 15, 2016 Passing Peony and Iris Plants on from Generation to Generation Annette Meyer Heisdorffer Daviess County Extension Agent for Horticulture
May 15, 2016 Passing Peony and Iris Plants on from Generation to Generation Annette Meyer Heisdorffer Daviess County Extension Agent for Horticulture After lunch on Mother’s Day, my mom and I surveyed her garden, especially the peonies. We both agreed that I needed to propagate her peonies and plant them in my garden. These are special, because I remember them growing in my grandmother’s garden. Peonies are commonly passed down from generation to generation. My goal is to someday share them with my twins. Our discussion included the irises, which are another heritage plant. Both plants are blooming beautifully in May and are spectacular in the garden. Information about these two plants will be provided in this article. Peony (Paeonia officinalis, Paeonia lactiflora, and hybrids) is a herbaceous perennial, which means at the end of the growing season it will die back to the ground. However, the plant returns year after year. Peonies grow best in full sun and well-drained soil. There are tree peonies (Paeonia suffruticosa) which have a woody stem, but those are not as common and require different growing conditions. The tree peony will not be discussed here. According to Dr. Rick Durham, Extension Specialist for Consumer Horticulture, peonies can be found in landscapes across Kentucky. Peonies have a long life span and are commonly grown in the garden. When planting the root, make sure it is not too deep. The eyes or bud should be just below the surface of the soil. If it is planted too deeply, the plants won't bloom. -

Aeroponic and Hydroponic Systems for Medicinal Herb, Rhizome, and Root Crops Anita L
Aeroponic and Hydroponic Systems for Medicinal Herb, Rhizome, and Root Crops Anita L. Hayden1 Native American Botanics Corporation, P.O. Box 44287, Tucson, AZ 85733 Additional index words. Arctium, Urtica, Anemopsis, Zingiber, Scutellaria, greenhouse Summary. Hydroponic and aeroponic production of medicinal crops in controlled environments provides opportunities for improving quality, purity, consistency, bioactivity, and biomass production on a commercial scale. Ideally, the goal is to optimize the environment and systems to maximize all five characteristics. Examples of crop production systems using perlite hydropon- ics, nutrient film technique (NFT), ebb and flow, and aeroponics were studied for various root, rhizome, and herb leaf crops. Biomass data comparing aeroponic vs. soilless culture or field grown production of burdock root (Arctium lappa), stinging nettles herb and rhizome (Urtica dioica), and yerba mansa root and rhizome (Anemopsis californica) are presented, as well as smaller scale projects observing ginger rhizome (Zingiber officinale) and skullcap herb (Scutellaria lateriflora). Phytochemical concentration of marker compounds for burdock and yerba mansa in different growing systems are presented. Production of medicinal herb and root crops the plants hydrated. NFT is a gutter (channel) of the crop are suspended in a spray chamber in controlled environments (CE) provides op- system without any aggregate medium, and where they are fully accessible for monitoring portunities for improving the quality, purity, where the fertilizer -

Enzymatic Hydrolysis of Lotus Rhizome Starch Using Α-Amylase and Glucoamylase
Journal of Food and Nutrition Research (ISSN 1336-8672) Vol. 56, 2017, No. 4, pp. 372–380 Enzymatic hydrolysis of lotus rhizome starch using α-amylase and glucoamylase LI GUO Summary To study the susceptibility of lotus root starch to digestive enzymes and its potential impact on glycemic response, enzyme kinetics and in vitro digestibility of the granular, gelatinized and retrograded starches were analysed. The results showed that the digestion rate coefficient values of the granular, gelatinized and retrograded starches were 4.6 × 10-3 min-1, 9.8 × 10-3 min−1 and 2.3 × 10-3 min−1, respectively. Compared to the granular starch, content of rapid digestible starch (RDS) increased by 39.0 %, content of slowly digestible starch (SDS) and resistant starch (RS) decreased by 9.6 % and 15.0 % after gelatinization, respectively. While content of RDS decreased by 21.1 %, content of SDS and RS increased by 2.1 % and 20.8 % after retrogradation, respectively. As for glycemic index (GI) and hydroly- sis index (HI), GI (70.57) and HI (56.21) of the gelatinized starch were higher than GI (66.63) and HI (49.03) of the granular starch, and GI (57.83) and HI (33.01) of the retrograded starch. The results provide an interesting information about exploring novel and slow digestible foods made of lotus root starch for potential health benefits. Keywords lotus root starch; digestibility; α-amylase; glucoamylase Lotus (Nelumbo nucifera) is a well-known and mucosal α-glucosidase in the human gastroin- medicinal plant widely cultivated in Asian coun- testinal tract [5, 6]. -

Basic Guide to the Culture of Peonies This Is Easily Done by Pulling Them Downwards and Sideways with the Fingers
A handful of sheep manure to a plant may be given in the spring to improve the bloom. Liquid manure also may be used with discretion, for the same purpose. Disbudding. Most varieties of peonies develop several small lateral or America’s Largest Grower of Daylilies, Peonies and Iris sidebuds near the base of the terminal bud. If large flowers are wanted, the side buds should be removed so the strength will all go into the terminal bud. The side-buds should be removed as soon as they are about the size of a pea. Basic Guide to the Culture of Peonies This is easily done by pulling them downwards and sideways with the fingers. o Some people prefer leaving their side-buds which develop and prolong the P.O. Box 338 • Sarcoxie, MO 64862 • (417) 548-3514 blooming season. The side-buds bloom later than the terminal buds. Insect Pests. In some sections of the country, where thrips are Basic Guide to the Culture of Peonies prevalent, some late varieties are damaged to the extent that the buds fail to open even after they are almost fully developed. Spraying or dusting, at Peonies are easily grown and their requirements are few, but they respond weekly intervals should control the thrips very well. Apply first application beautifully to a little special care and attention by producing best quality when buds are about the size of a large marble. We like Orthene. flowers and many of them. With this thought in mind we offer the following suggestions gained from many years experience in growing and Planted too deeply...examine and if Why Peonies Do Not Bloom. -

STEVEN and ERIN FORD GARDEN Botanical Name Common Name
STEVEN AND ERIN FORD GARDEN Botanical Name Common Name Abutilon Flowering Maple Acer buergerianum Trident Maple Acer japonicum Full Moon Maple Acer palmatum Japanese Maple Acer palmatum 'Bloodgood' Japanese Maple Achillea millefolium Yarrow Adiantum Maidenhair Fern Agapanthus Lily of the Nile Ajuga Alcea rosea Ajuga Alcea rosea Hollyhock Anemone japonica Japanese Anemone Antirrhinum majus Snapdragon Aquilegia Columbine Aralia Japanese Aralia Artemisia stelleriana Dusty Miller Artemisia stelleriana Powis Castle Artemisia Asparagus densiflorus Asparagus Fern Asparagus densiflorus 'Sprengeri' Sprenger Asparagus Fern Asparagus retrofractus Ming Fern Azalea Azalea Azalea 'Hino' Basil Begonia Berberis thumbergii 'Golden Nugget' Japanese Barberry Bletilla striata Chinese Ground Orchid Boysenberries Buddleja Butterfly Bush Buxus sempervirens Common Boxwood Calendula officinalis Pot Marigold Camellia japonica Camellia sasanqua Carex oshimensis Sedge Carex variegata Sedge Caryopteris Bluebird Ceanothus Cedrus deodara Deodar Cedar Centranthus ruber Jupiter's Beard Cercis canadensis Eastern Rudbud Chives Chrysanthemum Chrysanthemum maximum Shasta Daisy Citrus Dwarf Blood Orange Citrus Dwarf Grapefruit 1 Citrus Dwarf Tangerine Citrus Navel Orange Citrus Variegated Lemon Clematis Cornus Dogwood Cornus kousa Kousa Dogwood Cornus stolonifera Redtwig Dogwood Cotinus Smoke Tree Cryptomeria japonica Japanese Cryptomeria Cyathea cooperi Australian Tree Fern Cyclamen Delphinium Dianella tasmanica 'Yellow Stripe' Flax Lily Dianthus Pink Dianthus barbatus Sweet -

Culture of Iris Anne M
G1741 Culture of Iris Anne M. Streich, Extension Educator Dale T. Lindgren, Horticulture Specialist Iris culture emphasizes the best in site selection and preparation, planting, culture, and insect and dis- ease control. Irises are among the most popular and beautiful garden flowers for Midwest landscapes (Figure 1). More than 200 species of irises have been found in the wild and from these species, thousands of varieties have been named and made available for public use. Iris plants range in height from just a few inches to over 3 feet and are adapted to a variety of environmental conditions. The standard iris, Japanese iris, Siberian iris, Spuria and yellowflag types are suitable for Nebraska. Iris flowers can be from 1 or 2 inches across up to 8 to Figure 1. Irises 10 inches across and come in almost every color and often in two-color combinations. Irises can be selected to have continuous flowering from early April through June by using Planting an assortment of iris species and cultivars. Irises can be divided into “bearded” and “beardless” Irises grow from an enlarged underground stem called types. The term “bearded” refers to the presence of bushy a rhizome. These rhizomes grow just below the soil surface. “beards” on each of three drooping, petal-like sepals, called They are the source of growth for fans of leaves, flowers falls. The true petals are called standards and are upright. and the roots that anchor the plant. Rhizomes are used to Bearded irises, commonly called standard irises, are the most vegetatively propagate new plants of the same type. -

Patterns in Evolution in Characters That Define Iris Subgenera And
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 34 2006 Patterns in Evolution in Characters That Define rI is Subgenera and Sections Carol A. Wilson Rancho Santa Ana Botanic Garden Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Wilson, Carol A. (2006) "Patterns in Evolution in Characters That Define rI is Subgenera and Sections," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 34. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/34 Aliso 22, pp. 425-433 © 2006, Rancho Santa Ana Botanic Garden PATTERNS OF EVOLUTION IN CHARACTERS THAT DEFINE IRIS SUBGENERA AND SECTIONS CAROL A. WILSON Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711-3157, USA (carol. wilson@ cgu. edu) ABSTRACT Subgeneric groups have been circumscribed in Iris based on a small number of morphological characters. Recent DNA sequence data has indicated that several of the subgenera, sections, and series that have previously been delineated are paraphyletic or polyphyletic. The evolution of characters that have traditionally been used to distinguish sub generic and sectional groups within Iris was investigated by mapping these characters on a phylogenetic tree based on matK sequence data. Results indicate that rhizomes are pleisomorphic for the genus and that three bulb types have arisen independently. My analysis shows that sepal beards, sepal crests, and seed arils show extensive homoplasy. Most of the homoplasy seen is associated with the circumscription of polyphyletic subgeneric groups such as the beardless subgenus Limniris. Some additional homoplasy is due to diversity within supported clades or the historical use of a single character in circumscribing more than one subgeneric group. -

The Impacts of the Entanglement Concentration on the Hydrodynamic Properties of Kudzu and Lotus Rhizome Starch Aqueous Solutions
Journal of Food and Nutrition Research, 2016, Vol. 4, No. 11, 750-759 Available online at http://pubs.sciepub.com/jfnr/4/11/8 ©Science and Education Publishing DOI:10.12691/jfnr-4-11-8 The Impacts of the Entanglement Concentration on the Hydrodynamic Properties of Kudzu and Lotus Rhizome Starch Aqueous Solutions Li Guo*, Shuilin Wang, Chenchen Zhu, Jian Hu, Juanjuan Zhang, Xianfeng Du* Department of Food Sciences, Anhui Agricultural University, Hefei, China *Corresponding author: [email protected]; [email protected] Abstract With the rapid development of consumer demands for health, kudzu and lotus rhizome starches have been widely utilized as the nutritiously and naturally medicinal drinks after they are suspended in aqueous solutions. However, it is difficult to control the suitable concentrations to obtain the ideal textures of the kudzu and lotus rhizome starch solutions. In this study, on the basis of starch structure characteristics, the hydrodynamic properties of the kudzu and lotus rhizome starch aqueous solutions around entanglement concentration (the boundary between the semi-dilute regime and the concentrated regime of a polymer solution, ce) were studied. The results indicated that the two starch solutions showed a clear up-turn curve of the ηsp/c versus c curves in dilute solutions. The ce values of the kudzu and lotus rhizome starch aqueous solutions were determined to be 1.56% and 0.6%, respectively. The impact of the ce value on the network formation of the kudzu starch solutions was much more significant compared with the impact on the lotus rhizome starch solutions. Shear thinning behaviour hardly occurs when the concentrations of the kudzu and lotus rhizome starch aqueous solutions were lower than ce, and shear thinning behaviour develops when the concentrations are equal to or greater than ce. -

Best Plants for Problem Clay Soils: Perennials
Visit us on the Web: www.gardeninghelp.org Best Plants for Problem Clay Soils: Perennials Perennials Amsonia tabernaemontana — Bluestar This Missouri native features uptight clusters of light blue star-like flowers in late spring. Its narrow willow-like leaves turn yellow to peach-colored in fall. Bluestar may require staking if grown in shade and may be pruned after flowering to maintain a compact shape. It is most attractive when grown massed, in native plant gardens, shade gardens, open woodland areas, and borders. Asclepias incarnata — Swamp milkweed Despite its common name and native habitat, swamp milkweed may be grown in the average garden. Its fragrant white, pink or mauve flowers attract butterflies and mature into slender pods with silky-haired seeds. Swamp milkweed is a good choice for sunny, low or moist areas such as stream or pond banks, borders, and butterfly gardens. Baptisia australis — Blue false indigo Blue false indigo has beautiful purplish blue lupine-like flowers borne in erect spikes above the trifoliate leaves. The flowers mature into black seed pods that rattle in the breeze and are an interesting addition to dried flower arrangements. This herbaceous perennial does best in full sun as plants grown in part shade may grow taller and need support. Due to an extensive root system, blue false indigo will tolerate drought, but it should not be disturbed once it is established. Attractive in almost any situation including borders, prairies, cottage gardens, and native plant gardens, this plant is best used as a single specimen plant or in small groups. Baptisia australis var. -

Rhizome Elongation and Seagrass Clonal Growth
MARINE ECOLOGY PROGRESS SERIES Published November 26 Mar Ecol Prog Ser Rhizome elongation and seagrass clonal growth Nuria Marball*, Carlos M. ~uarte* 'Centre for Estuarine and Coastal Ecology, NIOO, Korringaweg 7, 4401 NT Yerseke. The Netherlands 2~entred'Estudis Avanqats de Blanes, CSIC, Cami de Sta. Barbara sln, E-17300 Blanes, Spain ABSTRACT. A compilation of published and original data on rhizome morphometry, horizontal and vertical elongation rates and branching patterns for 27 seagrass species developing in 192 seagrass stands allowed an examination of the variability of seagrass rhizome and clonal growth programmes across and within species. Seagrass horizontal rhizomes extend at rates ranging between 1.2 and 574 cm yr-l, develop a branch, with an angle from 19 to 72", for every 6 to 1800 horizontal internodes, and add a new shoot for every 1.1 to 7.5 cm of rhizome produced. Vertical rhizomes elongate at rates between 0.1 and 34 cm yr-' and the probability that they will branch varies over 3 orders of magnitude. Much (between 40 and 173%) of the variability of seagrass horizontal rhizome and clonal growth pro- grammes is species-specific, largely (21 to 63% of the variance) associated with differences in size among species, although seagrasses also show important intraspecific variability. The broad repertoire of seagrass rhizome and clonal growth programmes explains the different rates and efficiency at which the species occupy space. The implications of specific growth programmes for space occupation were examined by simulating the development of seagrass rhizome networks of 3 seagrass species encom- passing the range of horizontal rhizome growth [Halophila ovalis, Thalassodendron ciliaturn, Posidonia oceanica).