Anti-D (Series 4) Monoclonal Blend Blood Grouping Reagent for Slide, Tube and Microplate Tests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oristm Cell Migration Assay – Tricoated
TM Oris Cell Migration Assay – TriCoated Product No.: CMATR1 & CMATR5 96-well, 2-D Assay for Investigating Cell Migration of Adherent Cell Lines on Three Coated Surfaces PROTOCOL & INSTRUCTIONS Table of Contents I. INTRODUCTION .................................................................................................. 2 II. ORISTM PLATE DIMENSIONS .............................................................................. 3 III. MATERIALS PROVIDED ...................................................................................... 3 IV. MATERIALS REQUIRED ...................................................................................... 3 V. CELL MIGRATION ASSAY – TRICOATED PROTOCOL .................................... 4 VI. DATA ACQUISITION ............................................................................................ 6 VII. ORDERING INFORMATION ................................................................................ 7 VIII. TERMS & CONDITIONS ...................................................................................... 7 Appendix I: Determining Optimal Cell Seeding Concentration ............................. 8 Appendix II: Determining Optimal Fluorescence Microplate Reader Settings ...... 8 Platypus Technologies, LLC 5520 Nobel Drive, Suite 100, Madison, WI 53711 Toll Free: 866.3296.4455 Phone: 608.237.1270 Fax: 608.237.1271 www.platypustech.com SP0034.06 Oris™ CELL MIGRATION ASSAY – TRICOATED I. INTRODUCTION The Oris™ Cell Migration Assay – TriCoated is a reproducible, sensitive, and flexible -

Determination of the Identity of an Unknown Liquid Group # My Name the Date My Period Partner #1 Name Partner #2 Name
Determination of the Identity of an unknown liquid Group # My Name The date My period Partner #1 name Partner #2 name Purpose: The purpose of this lab is to determine the identity of an unknown liquid by measuring its density, melting point, boiling point, and solubility in both water and alcohol, and then comparing the results to the values for known substances. Procedure: 1) Density determination Obtain a 10mL sample of the unknown liquid using a graduated cylinder Determine the mass of the 10mL sample Save the sample for further use 2) Melting point determination Set up an ice bath using a 600mL beaker Obtain a ~5mL sample of the unknown liquid in a clean dry test tube Place a thermometer in the test tube with the sample Place the test tube in the ice water bath Watch for signs of crystallization, noting the temperature of the sample when it occurs Save the sample for further use 3) Boiling point determination Set up a hot water bath using a 250mL beaker Begin heating the water in the beaker Obtain a ~10mL sample of the unknown in a clean, dry test tube Add a boiling stone to the test tube with the unknown Open the computer interface software, using a graph and digit display Place the temperature sensor in the test tube so it is in the unknown liquid Record the temperature of the sample in the test tube using the computer interface Watch for signs of boiling, noting the temperature of the unknown Dispose of the sample in the assigned waste container 4) Solubility determination Obtain two small (~1mL) samples of the unknown in two small test tubes Add an equal amount of deionized into one of the samples Add an equal amount of ethanol into the other Mix both samples thoroughly Compare the samples for solubility Dispose of the samples in the assigned waste container Observations: The unknown is a clear, colorless liquid. -

Increasing the Reproducibility of Serial Dilutions
Increasing the Reproducibility of Serial Dilutions Michael Beier* Abstract Pipetting serial dilutions reproducibly can be difficult because it is a time consuming and consequently error prone methodology, especially in 384 well plates. Based upon the assumption that your pipettes are properly maintained and calibrated, the human influence has the largest impact on pipetting results. Observing good pipetting practice helps immensely to improve reproducibility and accuracy. In this article we focus on a selection of good pipetting techniques, which if maintained offer the best chance of increasing and maintaining the reproducibility of your serial dilutions. Mixing is a key component of dilution protocols and in this article we additionally discuss different mixing parameters to help you achieve a homogenous sample. To maximize consistency of dilution assays, automating the process offers many benefits. For ensuring highly reproducible serial dilutions we outline what features an automated pipetting system should have and introduce the VIAFLO ASSIST – a product purpose designed for this task. Introduction to good pipetting practice Good pipetting practice describes guidelines covering the basic techniques to ensure that the pipettes are used in an optimal and reproducible manner. Before preparing your assay, you should perform a pre-wet of your pipette tips. Do this by aspirating and dispensing the full volume 2-3 times. This procedure not only equilibrates temperature differences between liquid and tip but also humidifies the dead air space inside the pipette and tip. Neglecting to perform a pre-wet can result in a smaller delivery volume in the first few dispenses, thereby introducing errors into your subsequent dilution steps. -

Evos Cell Imaging Analysis Systems
Imaging, labeling, and detection solutions Microscopy | High-content analysis | Cell counting | Plate reading Minimizing the complexities of cellular analysis Our cellular analysis product portfolio combines the strengths of Invitrogen™ fluorescent reagents and a complete line of versatile analysis instrumentation. Select from a line of heavily peer-referenced platforms to make the discoveries that catalyze advances toward your research goals of tomorrow. Invitrogen™ EVOS™ imaging systems Our comprehensive imaging portfolio includes: • Cell imaging systems • Automated cell counting systems • High-content analysis systems Invitrogen™ Countess™ cell counters • Cell imaging reagents • Microplate readers All of our analysis systems are designed to work together— Thermo Scientific™ CellInsight™ from the initial cell culture check to more complex analyses. high-content analysis systems Discover more about your samples with automated cell counting, long-term live-cell imaging, automated multiwell plate scanning, and phenotypic screening. Thermo Scientific™ Varioskan™ LUX multimode plate reader Contents Microscopy Compact and portable imaging systems 4 EVOS imaging systems at a glance 5 EVOS M7000 Imaging System 6 Live-cell imaging with the Onstage Incubator 8 Image analysis with Celleste software 10 EVOS M5000 Imaging System 12 EVOS FLoid Imaging Station 14 EVOS XL Core Imaging System 15 EVOS vessel holders and stage plates 16 The power of LED illumination 17 EVOS objectives 18 Fluorophore selection guide 20 High-content analysis CellInsight high-content analysis platforms 22 CellInsight CX7 LZR High-Content Analysis Platform 24 CellInsight CX5 High-Content Screening Platform 25 HCS Studio Cell Analysis Software 26 Store Image and Database Management Software 27 Cell counting Countess II Automated Cell Counters 28 Plate reading Microplate readers 30 Microscopy Compact and portable imaging systems Now you can have an easy-to-use cell imaging platform From intimate hands-on demonstrations to presentations of where you want it and when you want it. -

ELISA Plate Reader
applications guide to microplate systems applications guide to microplate systems GETTING THE MOST FROM YOUR MOLECULAR DEVICES MICROPLATE SYSTEMS SALES OFFICES United States Molecular Devices Corp. Tel. 800-635-5577 Fax 408-747-3601 United Kingdom Molecular Devices Ltd. Tel. +44-118-944-8000 Fax +44-118-944-8001 Germany Molecular Devices GMBH Tel. +49-89-9620-2340 Fax +49-89-9620-2345 Japan Nihon Molecular Devices Tel. +06-6399-8211 Fax +06-6399-8212 www.moleculardevices.com ©2002 Molecular Devices Corporation. Printed in U.S.A. #0120-1293A SpectraMax, SoftMax Pro, Vmax and Emax are registered trademarks and VersaMax, Lmax, CatchPoint and Stoplight Red are trademarks of Molecular Devices Corporation. All other trademarks are proprty of their respective companies. complete solutions for signal transduction assays AN EXAMPLE USING THE CATCHPOINT CYCLIC-AMP FLUORESCENT ASSAY KIT AND THE GEMINI XS MICROPLATE READER The Molecular Devices family of products typical applications for Molecular Devices microplate readers offers complete solutions for your signal transduction assays. Our integrated systems γ α β s include readers, washers, software and reagents. GDP αs AC absorbance fluorescence luminescence GTP PRINCIPLE OF CATCHPOINT CYCLIC-AMP ASSAY readers readers readers > Cell lysate is incubated with anti-cAMP assay type SpectraMax® SpectraMax® SpectraMax® VersaMax™ VMax® EMax® Gemini XS LMax™ ATP Plus384 190 340PC384 antibody and cAMP-HRP conjugate ELISA/IMMUNOASSAYS > nucleus Single addition step PROTEIN QUANTITATION cAMP > λEX 530 nm/λEM 590 nm, λCO 570 nm UV (280) Bradford, BCA, Lowry For more information on CatchPoint™ assay NanoOrange™, CBQCA kits, including the complete procedure for this NUCLEIC ACID QUANTITATION assay (MaxLine Application Note #46), visit UV (260) our web site at www.moleculardevices.com. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

LOWERING COSTS and SIMPLIFYING WORKFLOW with the Automate 2550 SYSTEM
HACETTEPE UNIVERSITY FACULTY OF MEDICINE HOSPITAL CENTRAL AND STAT LABORATORIES LOWERING COSTS AND SIMPLIFYING WORKFLOW WITH THE AutoMate 2550 SYSTEM Laboratory profile › 1,040-bed hospital located in Ankara, Turkey › Provides uninterrupted 24/7 inpatient, outpatient and emergency services › Employs 6 professors, 6 associate Hacettepe University Faculty of Medicine Hospital is one of the leading professors and 130 operators university hospitals in Turkey. The university is well known for its › Performs up to 6.5 million geographical location and adoption of ground breaking technologies. biochemistry and hormone While the reference site is preferred by a large number of patients, it is tests annually of particular interest to specific patient populations seeking test results › Processes up to 2 million tests from a variety of panels. With many different systems performing on instruments in more than hundreds of standard and specialized tests, the hospital’s laboratories 20 different units are driven to ensure the delivery of results without error, while › Operates a Beckman Coulter containing costs and managing workload burden. standalone AutoMate 2550 pre- For more than 16 years, the facility—which has been held up as an and post-analytical automation system in the biochemistry example of excellence in its own region, as well as all over the country— laboratory, along with a Power has been using a standalone automation system with an aliquoter Processor system with two feature. With this technology, it has enhanced patient satisfaction, -
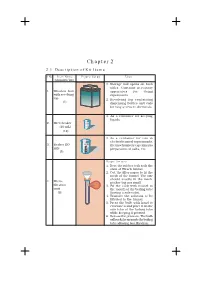
Chapter 2 2.1 Description of Kit Items
Manual of SEAT Friendly MCL Kit 7 Chapter 2 2.1 Description of Kit Items S.No. Item Name Figure/Setup Uses (Quantity/ kit) 1. Storage box opens on both sides. Contains necessary 1. Wooden box apparatus for doing with revolving experiments. top 2. Revolving top containing (1) dispensing bottles and vials for easy access to chemicals. 1. As a container for keeping liquids. 2. Microbeaker (10 mL) (12) 1. As a container for use in electrochemical experiments, 3. Beaker (50 thermochemistry experiments mL) preparation of salts, etc. (3) Steps for use 1. Bore the rubber cork to fit the stem of Hirsch funnel. 2. Cut the filter paper to fit the mesh of the funnel. The size should exactly fit the mesh, 4. Micro- neither big nor small. filtration 3. Fit the cork with funnel in unit the mouth of the boiling tube (2) (having a side tube). 4. Transfer the solution to be filtrated to the funnel. 5. Press the bulb with hand to evacuate it and place it on the side tube of the boiling tube while keeping it pressed. 6. Release the pressure. The bulb will suck the air inside the boiling tube allowing fast filtration. 8 Manual of SEAT Friendly MCL Kit S.No. Item Name Figure/Setup Uses (Quantity/Kit) 1. The watch glass is used to hold solids when being 5. Watch glass weighed. (2) 2. It should never be heated. 1. Flat plate with multiple “wells” used as micro test tubes. 2. Contains 96 rectangular matrix. 3. Each well of a micro plate typically holds somewhere between a few to a few hundred microlitres of liquid. -

Wellwash and Wellwash Versa Washers for Expanded Functionality Ease of Use, Convenience, and Versatility for Washing Microplates in ELISA Assays
Wellwash and Wellwash Versa washers for expanded functionality Ease of use, convenience, and versatility for washing microplates in ELISA assays Reliable and easy-to-use Thermo Scientifi ™c Wellwash™ microplate strip washers provide secure washing performance for routine and research ELISA applications. Wellwash washers feature a large color display with graphical user interface, local language versions, and non-pressurized bottles for optimal ease of use, convenience, and safety. The Thermo Scientifi ™c Wellwash™ Microplate Washer is the In addition, the Wellwash Versa washer off ers: basic model for washing 96-well plates. It is intended for • Very fast and simple instrument setup and use with use when only a few similar assays are run routinely. The color-coded tube fi ttings and liquid-level sensor cables Thermo Scientifi ™c Wellwash™ Versa Microplate Washer is the advanced model for 96-well plates that can also wash • Wide selection of wash heads for 96-well plates and cells and 384-well plates, off ering the enhanced fl exibility 1 x 16 wash head for 384-well plates needed for research use. • Specially designed 2 x 8-cell wash head for gentle washing of cells in a 96-well plate Wellwash and Wellwash Versa washers off er: • Easy setup of wash protocols through a large color • Two wash bottles and one rinse bottle for screen and a visual user interface increased fl exibility • A sweep mode to ensure an extremely low residual • Easy connection to automation systems volume in the well, resulting in excellent washing performance and reliable ELISA results Convenient and simple to use • Adjustable wash parameters, such as dispense and The large color screen makes Wellwash and Wellwash aspiration height and aspiration speed, for optimal Versa washers very easy and convenient to use. -

Laboratory Supplies and Equipment
Laboratory Supplies and Equipment Beakers: 9 - 12 • Beakers with Handles • Printed Square Ratio Beakers • Griffin Style Molded Beakers • Tapered PP, PMP & PTFE Beakers • Heatable PTFE Beakers Bottles: 17 - 32 • Plastic Laboratory Bottles • Rectangular & Square Bottles Heatable PTFE Beakers Page 12 • Tamper Evident Plastic Bottles • Concertina Collapsible Bottle • Plastic Dispensing Bottles NEW Straight-Side Containers • Plastic Wash Bottles PETE with White PP Closures • PTFE Bottle Pourers Page 39 Containers: 38 - 42 • Screw Cap Plastic Jars & Containers • Snap Cap Plastic Jars & Containers • Hinged Lid Plastic Containers • Dispensing Plastic Containers • Graduated Plastic Containers • Disposable Plastic Containers Cylinders: 45 - 48 • Clear Plastic Cylinder, PMP • Translucent Plastic Cylinder, PP • Short Form Plastic Cylinder, PP • Four Liter Plastic Cylinder, PP NEW Polycarbonate Graduated Bottles with PP Closures Page 21 • Certified Plastic Cylinder, PMP • Hydrometer Jar, PP • Conical Shape Plastic Cylinder, PP Disposal Boxes: 54 - 55 • Bio-bin Waste Disposal Containers • Glass Disposal Boxes • Burn-upTM Bins • Plastic Recycling Boxes • Non-Hazardous Disposal Boxes Printed Cylinders Page 47 Drying Racks: 55 - 56 • Kartell Plastic Drying Rack, High Impact PS • Dynalon Mega-Peg Plastic Drying Rack • Azlon Epoxy Coated Drying Rack • Plastic Draining Baskets • Custom Size Drying Racks Available Burn-upTM Bins Page 54 Dynalon® Labware Table of Contents and Introduction ® Dynalon Labware, a leading wholesaler of plastic lab supplies throughout -

High School Chemistry
RECOMMENDED MINIMUM CORE INVENTORY TO SUPPORT STANDARDS-BASED INSTRUCTION HIGH SCHOOL GRADES SCIENCES High School Chemistry Quantity per Quantity per lab classroom/ Description group adjacent work area SAFETY EQUIPMENT 2 Acid storage cabinet (one reserved exclusively for nitric acid) 1 Chemical spill kit 1 Chemical storage reference book 5 Chemical waste containers (Categories: corrosives, flammables, oxidizers, air/water reactive, toxic) 1 Emergency shower 1 Eye wash station 1 Fire blanket 1 Fire extinguisher 1 First aid kit 1 Flammables cabinet 1 Fume hood 1/student Goggles 1 Goggles sanitizer (holds 36 pairs of goggles) 1/student Lab aprons COMPUTER ASSISTED LEARNING 1 Television or digital projector 1 VGA Adapters for various digital devices EQUIPMENT/SUPPLIES 1 box Aluminum foil 100 Assorted rubber stoppers 1 Balance, analytical (0.001g precision) 5 Balance, electronic or manual (0.01g precision) 1 pkg of 50 Balloons, latex 4 Beakers, 50 mL 4 Beakers, 100 mL 2 Beakers, 250 mL Developed by California Science Teachers Association to support the implementation of the California Next Generation Science Standards. Approved by the CSTA Board of Directors November 17, 2015. Quantity per Quantity per lab classroom/ Description group adjacent work area 2 Beakers, 400 or 600 mL 1 Beakers, 1000 mL 1 Beaker tongs 1 Bell jar 4 Bottle, carboy round, LDPE 10 L 4 Bottle, carboy round, LDPE 4 L 10 Bottle, narrow mouth, 1000 mL 20 Bottle, narrow mouth, 125 mL 20 Bottle, narrow mouth, 250 mL 20 Bottle, narrow mouth, 500 mL 10 Bottle, wide mouth, 125 -

Modulus™ II Microplate Multimode Reader
Modulus™ II Microplate Multimode Reader Operating Manual Part Number 998-9375 Rev E Turner BioSystems, Inc. and its suppliers own the written and other materials in the Modulus II Microplate Reader operating manual. The written materials are copyrighted and protected by the copyright laws of the United States of America and, throughout the world, by the copyright laws of each jurisdiction according to pertinent treaties and conventions. No part of this manual may be reproduced manually, electronically, or in any other way without the written permission of Turner BioSystems, Inc. Modulus™ is a trademark of Turner BioSystems, Inc. All of the trademarks, service marks, and logos used in these materials are the property of Turner BioSystems, Inc. or their respective owners. All goods and services are sold subject to the terms and conditions of sale of the company within the Turner BioSystems, Inc. group that supplies them. A copy of these terms and conditions is available on request. NOTICE TO PURCHASER: The Modulus™ II Microplate Reader is for research purposes only. It is not intended or approved for diagnosis of disease in humans or animals. © Turner BioSystems, Inc. 2008 - All rights reserved. Made in the USA 2 Table of Contents 1 Instrument ............................................................................................................................... 7 1.1 Getting Started ................................................................................................................. 7 1.1.1 Description ...........................................................................................................