Therapeutic Options in Hereditary Optic Neuropathies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dual Role of the Mitochondrial Protein Frataxin in Astrocytic Tumors
Laboratory Investigation (2011) 91, 1766–1776 & 2011 USCAP, Inc All rights reserved 0023-6837/11 $32.00 Dual role of the mitochondrial protein frataxin in astrocytic tumors Elmar Kirches1, Nadine Andrae1, Aline Hoefer2, Barbara Kehler1,3, Kim Zarse4, Martin Leverkus3, Gerburg Keilhoff5, Peter Schonfeld5, Thomas Schneider6, Annette Wilisch-Neumann1 and Christian Mawrin1 The mitochondrial protein frataxin (FXN) is known to be involved in mitochondrial iron homeostasis and iron–sulfur cluster biogenesis. It is discussed to modulate function of the electron transport chain and production of reactive oxygen species (ROS). FXN loss in neurons and heart muscle cells causes an autosomal-dominant mitochondrial disorder, Friedreich’s ataxia. Recently, tumor induction after targeted FXN deletion in liver and reversal of the tumorigenic phenotype of colonic carcinoma cells following FXN overexpression were described in the literature, suggesting a tumor suppressor function. We hypothesized that a partial reversal of the malignant phenotype of glioma cells should occur after FXN transfection, if the mitochondrial protein has tumor suppressor functions in these brain tumors. In astrocytic brain tumors and tumor cell lines, we observed reduced FXN levels compared with non-neoplastic astrocytes. Mitochondrial content (citrate synthase activity) was not significantly altered in U87MG glioblastoma cells stably overexpressing FXN (U87-FXN). Surprisingly, U87-FXN cells exhibited increased cytoplasmic ROS levels, although mitochondrial ROS release was attenuated by FXN, as expected. Higher cytoplasmic ROS levels corresponded to reduced activities of glutathione peroxidase and catalase, and lower glutathione content. The defect of antioxidative capacity resulted in increased susceptibility of U87-FXN cells against oxidative stress induced by H2O2 or buthionine sulfoximine. -

Study Protocol
EspeRare_RIM_001 Rimeporide in DMD patients Page 1 of 85 Clinical Study Protocol Study title: A phase Ib, open label study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of multiple ascending oral doses of Rimeporide in patients with Duchenne Muscular Dystrophy Acronym: Rim4DMD Sponsor: EspeRare Foundation 14 Chemin des Aulx 1228 Plan-les-Ouates Switzerland Study Sponsor’s number: ESPERARE_RIM_001 EudraCT number: 2015-002530-50 Version: 2.1 Date: February 19th, 2016 Amendment number: 2 This document is a confidential communication of EspeRare. Acceptance of this document constitutes the agreement by the recipient that no unpublished information contained within will be published or disclosed without prior written approval, except as required to permit review by responsible Ethics Committees and Health Authorities or to obtain informed consent from potential patients. EudraCT Number 2015-002530-50 CONFIDENTIAL [Rim4DMD] – Final Version 2.1_ February 19th, 2016 EspeRare_RIM_001 Rimeporide in DMD patients Page 2 of 85 Investigator Agreement Protocol Number: EspeRare_RIM_001 EudraCT Number: 2015-002530-50 Protocol version and date: 2.1._ February 19th 2016 Sponsor: EspeRare Foundation Study drug: Rimeporide. Study title: A phase Ib, open label study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of multiple ascending oral doses of rimeporide in patients with Duchenne Muscular Dystrophy. Acronym: Rim4DMD. Investigator endorsement: I, the undersigned, am responsible for the conduct of this study at this site and agree to conduct the study according to the protocol and any approved protocol amendments, study specific procedures, all applicable laws and regulatory authority requirements including but not limited to European directives, ICH Good Clinical Practice (GCP), the Ethical principles that have their origins in the Declaration of Helsinki and applicable privacy laws. -

The Pathogenetic Role of Β-Cell Mitochondria in Type 2 Diabetes
236 3 Journal of M Fex et al. Mitochondria in β-cells 236:3 R145–R159 Endocrinology REVIEW The pathogenetic role of β-cell mitochondria in type 2 diabetes Malin Fex1, Lisa M Nicholas1, Neelanjan Vishnu1, Anya Medina1, Vladimir V Sharoyko1, David G Nicholls1, Peter Spégel1,2 and Hindrik Mulder1 1Department of Clinical Sciences in Malmö, Unit of Molecular Metabolism, Lund University Diabetes Centre, Clinical Research Center, Malmö University Hospital, Lund University, Malmö, Sweden 2Department of Chemistry, Center for Analysis and Synthesis, Lund University, Sweden Correspondence should be addressed to H Mulder: [email protected] Abstract Mitochondrial metabolism is a major determinant of insulin secretion from pancreatic Key Words β-cells. Type 2 diabetes evolves when β-cells fail to release appropriate amounts f TCA cycle of insulin in response to glucose. This results in hyperglycemia and metabolic f coupling signal dysregulation. Evidence has recently been mounting that mitochondrial dysfunction f oxidative phosphorylation plays an important role in these processes. Monogenic dysfunction of mitochondria is a f mitochondrial transcription rare condition but causes a type 2 diabetes-like syndrome owing to β-cell failure. Here, f genetic variation we describe novel advances in research on mitochondrial dysfunction in the β-cell in type 2 diabetes, with a focus on human studies. Relevant studies in animal and cell models of the disease are described. Transcriptional and translational regulation in mitochondria are particularly emphasized. The role of metabolic enzymes and pathways and their impact on β-cell function in type 2 diabetes pathophysiology are discussed. The role of genetic variation in mitochondrial function leading to type 2 diabetes is highlighted. -

Idebenone Protects Against Acute Murine Colitis Via Antioxidant and Anti-Inflammatory Mechanisms
International Journal of Molecular Sciences Article Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms Sonia Shastri 1,*, Tanvi Shinde 1,2 , Sukhwinder Singh Sohal 1, Nuri Gueven 3 and Rajaraman Eri 1,* 1 Department of Laboratory Medicine, School of Health Sciences, College of Health and Medicine, University of Tasmania, Launceston, Tasmania 7250, Australia; [email protected] (T.S.); [email protected] (S.S.S.) 2 Centre for Food Safety and Innovation, Tasmanian Institute of Agriculture, University of Tasmania, Launceston, Tasmania 7250, Australia 3 Pharmacy, School of Medicine, College of Health and Medicine, University of Tasmania, Hobart, Tasmania 7005, Australia; [email protected] * Correspondence: [email protected] (S.S.); [email protected] (R.E.); Tel.: +61-4-4992-4236 (S.S.); +61-3-6226-5017 (R.E.) Received: 3 December 2019; Accepted: 9 January 2020; Published: 12 January 2020 Abstract: Oxidative stress is a key player of the inflammatory cascade responsible for the initiation of ulcerative colitis (UC). Although the short chain quinone idebenone is considered a potent antioxidant and a mitochondrial electron donor, emerging evidence suggests that idebenone also displays anti-inflammatory activity. This study evaluated the impact of idebenone in the widely used dextran sodium sulphate (DSS)-induced mouse model of acute colitis. Acute colitis was induced in C57BL/6J mice via continuous exposure to 2.5% DSS over 7 days. Idebenone was co-administered orally at a dose of 200 mg/kg body weight. Idebenone significantly prevented body weight loss and improved the disease activity index (DAI), colon length, and histopathological score. -

Src Inhibitors Modulate Frataxin Protein Levels
Human Molecular Genetics, 2015, Vol. 24, No. 15 4296–4305 doi: 10.1093/hmg/ddv162 Advance Access Publication Date: 6 May 2015 Original Article ORIGINAL ARTICLE Src inhibitors modulate frataxin protein levels Downloaded from https://academic.oup.com/hmg/article/24/15/4296/2453025 by guest on 28 September 2021 Fabio Cherubini1, Dario Serio1, Ilaria Guccini1, Silvia Fortuni1,2, Gaetano Arcuri1, Ivano Condò1, Alessandra Rufini1,2, Shadman Moiz1, Serena Camerini3, Marco Crescenzi3, Roberto Testi1,2 and Florence Malisan1,* 1Laboratory of Signal Transduction, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Via Montpellier 1, 00133 Rome, Italy, 2Fratagene Therapeutics Ltd, 22 Northumberland Rd, Dublin, Ireland and 3Department of Cell Biology and Neurosciences, Italian National Institute of Health, Viale Regina Elena, 299, 00161 Rome, Italy *To whom correspondence should be addressed at: Laboratory of Signal Transduction, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Via Montpellier 1, 00133 Rome, Italy. Tel: +39 0672596501; Fax: +39 0672596505; Email: [email protected] Abstract Defective expression of frataxin is responsible for the inherited, progressive degenerative disease Friedreich’s Ataxia (FRDA). There is currently no effective approved treatment for FRDA and patients die prematurely. Defective frataxin expression causes critical metabolic changes, including redox imbalance and ATP deficiency. As these alterations are known to regulate the tyrosine kinase Src, we investigated whether Src might in turn affect frataxin expression. We found that frataxin can be phosphorylated by Src. Phosphorylation occurs primarily on Y118 and promotes frataxin ubiquitination, a signal for degradation. Accordingly, Src inhibitors induce accumulation of frataxin but are ineffective on a non-phosphorylatable frataxin- Y118F mutant. -
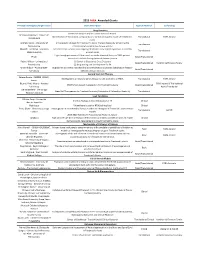
Grantsummaries 2014-2017 Jf
2015 FARA Awarded Grants Principal Investigator/Organization Grant Description Type of Research Co-funding Drug Discovery 2014 Keith Michael Andrus Cardiac Research Award: Veronique Monnier - Université Identification of therapeutic compounds on a cardiac Drosophila model of Friedreich’s Translational FARA Ireland Paris Diderot ataxia Andrew Dancis - University of A therapeutic strategy for Friedreich's ataxia: Frataxin bypass by enhancing the Translational Pennsylvania mitochondrial cysteine desulfurase activity Edward L. Grabczyk - Louisiana Small molecule induced exon skipping of MLH3 to slow repeat expansion in an FRDA Translational State University mousel model High throughput screen of Pfizer small molecule chemical library in FRDA patient- Pfizer Basic/Translational derived cells to look for regulators of frataxin protein Robert Wilson - University of (1) Center of Excellence: Drug Discovery Basic/Translational Hamilton & Finneran Family Pennsylvania (2) Drug testing and development for FA Omar Khdour - Arizona State Approaches to correct mitochondrial abnormalities and synaptic pathology in frataxin- Basic/Translational University deficient sensory neurons Gene & Stem Cell Therapy Helene Puccio - INSERM, IGBMC, Development of neuronal gene therapy for the treatment of FRDA Translational FARA Ireland France Ricardo Pinto Mouro - Harvard FARA Ireland & The National CRISPR/Cas9-based Therapeutics for Friedreich's ataxia Basic/Translational University Ataxia Foundation Joel Gottesfeld - The Scripps Stem Cell Therapeutics for Friedreich's -

Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’S Disease
biomedicines Review Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease Heng-Chung Kung 1,2 , Kai-Jung Lin 1,3 , Chia-Te Kung 4,* and Tsu-Kung Lin 1,5,6,* 1 Center for Mitochondrial Research and Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83301, Taiwan; [email protected] (H.-C.K.); [email protected] (K.-J.L.) 2 Department of Biology, Krieger School of Arts and Sciences, Johns Hopkins University, Baltimore, MD 21218, USA 3 Department of Family Medicine, National Taiwan University Hospital, Taipei 100225, Taiwan 4 Department of Emergency Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83301, Taiwan 5 Department of Neurology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83301, Taiwan 6 Center of Parkinson’s Disease, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 83301, Taiwan * Correspondence: [email protected] (C.-T.K.); [email protected] (T.-K.L.) Abstract: Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by dopaminergic neuronal loss. The exact pathogenesis of PD is complex and not yet completely understood, but research has established the critical role mitochondrial dysfunction plays in the development of PD. As the main producer of cytosolic reactive oxygen species (ROS), mito- chondria are particularly susceptible to oxidative stress once an imbalance between ROS generation and the organelle’s antioxidative system occurs. An overabundance of ROS in the mitochondria can Citation: Kung, H.-C.; Lin, K.-J.; lead to mitochondrial dysfunction and further vicious cycles. -

Strand Breaks for P53 Exon 6 and 8 Among Different Time Course of Folate Depletion Or Repletion in the Rectosigmoid Mucosa
SUPPLEMENTAL FIGURE COLON p53 EXONIC STRAND BREAKS DURING FOLATE DEPLETION-REPLETION INTERVENTION Supplemental Figure Legend Strand breaks for p53 exon 6 and 8 among different time course of folate depletion or repletion in the rectosigmoid mucosa. The input of DNA was controlled by GAPDH. The data is shown as ΔCt after normalized to GAPDH. The higher ΔCt the more strand breaks. The P value is shown in the figure. SUPPLEMENT S1 Genes that were significantly UPREGULATED after folate intervention (by unadjusted paired t-test), list is sorted by P value Gene Symbol Nucleotide P VALUE Description OLFM4 NM_006418 0.0000 Homo sapiens differentially expressed in hematopoietic lineages (GW112) mRNA. FMR1NB NM_152578 0.0000 Homo sapiens hypothetical protein FLJ25736 (FLJ25736) mRNA. IFI6 NM_002038 0.0001 Homo sapiens interferon alpha-inducible protein (clone IFI-6-16) (G1P3) transcript variant 1 mRNA. Homo sapiens UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 15 GALNTL5 NM_145292 0.0001 (GALNT15) mRNA. STIM2 NM_020860 0.0001 Homo sapiens stromal interaction molecule 2 (STIM2) mRNA. ZNF645 NM_152577 0.0002 Homo sapiens hypothetical protein FLJ25735 (FLJ25735) mRNA. ATP12A NM_001676 0.0002 Homo sapiens ATPase H+/K+ transporting nongastric alpha polypeptide (ATP12A) mRNA. U1SNRNPBP NM_007020 0.0003 Homo sapiens U1-snRNP binding protein homolog (U1SNRNPBP) transcript variant 1 mRNA. RNF125 NM_017831 0.0004 Homo sapiens ring finger protein 125 (RNF125) mRNA. FMNL1 NM_005892 0.0004 Homo sapiens formin-like (FMNL) mRNA. ISG15 NM_005101 0.0005 Homo sapiens interferon alpha-inducible protein (clone IFI-15K) (G1P2) mRNA. SLC6A14 NM_007231 0.0005 Homo sapiens solute carrier family 6 (neurotransmitter transporter) member 14 (SLC6A14) mRNA. -

Idebenone: Novel Strategies to Improve Its Systemic and Local Efficacy
nanomaterials Review Idebenone: Novel Strategies to Improve Its Systemic and Local Efficacy Lucia Montenegro 1,* ID , Rita Turnaturi 2 ID , Carmela Parenti 3 and Lorella Pasquinucci 2 ID 1 Department of Drug Sciences, Pharmaceutical Technology Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy 2 Department of Drug Sciences, Medicinal Chemistry Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy; [email protected] (R.T.); [email protected] (L.P.) 3 Department of Drug Sciences, Pharmacology and Toxicology Section, University of Catania, Viale A. Doria 6, 95125 Catania, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-095-738-4010 Received: 31 December 2017; Accepted: 30 January 2018; Published: 5 February 2018 Abstract: The key role of antioxidants in treating and preventing many systemic and topical diseases is well recognized. One of the most potent antioxidants available for pharmaceutical and cosmetic use is Idebenone (IDE), a synthetic analogue of Coenzyme Q10. Unfortunately, IDE’s unfavorable physicochemical properties such as poor water solubility and high lipophilicity impair its bioavailability after oral and topical administration and prevent its parenteral use. In recent decades, many strategies have been proposed to improve IDE effectiveness in the treatment of neurodegenerative diseases and skin disorders. After a brief description of IDE potential therapeutic applications and its pharmacokinetic and pharmacodynamic profile, this review will focus on the different approaches investigated to overcome IDE drawbacks, such as IDE incorporation into different types of delivery systems (liposomes, cyclodextrins, microemulsions, self-micro-emulsifying drug delivery systems, lipid-based nanoparticles, polymeric nanoparticles) and IDE chemical modification. -

213026Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 213026Orig1s000 CLINICAL REVIEW(S) Clinical Review David Hosford, Xiang Ling, Thomas Biel, Ashutosh Rao NDA 213,026: casimersen for DMD amenable to exon 45 skipping CLINICAL REVIEW Application Type NME, original NDA Application Number(s) NDA 213,026 (IND 118,086) Priority or Standard Priority Submit Date(s) 1/10/20 (submission 1 [clinical, nonclinical]) 6/25/20 (submission 2 [quality, complete NDA]) Received Date(s) 1/10/20; 6/25/20 PDUFA Goal Date 2/25/21 Division/Office DN1 / ON / OND / CDER Reviewer Name(s) David Hosford, ; Xiang Ling (Biometrics); Thomas Biel, Ashutosh Rao (Office of Biotechnology Products [OBP]) Review Completion Date 2/15/21 Established/Proper Name Casimersen (Proposed) Trade Name Amondys 45 Applicant Sarepta Therapeutics, Inc. (Cambridge, MA) Dosage Form(s) concentrated solution in a single use-vial for IV infusion Applicant Proposed Dosing 30mg/kg weekly Regimen(s) Applicant Proposed For the treatment of Duchenne muscular dystrophy (DMD) in Indication(s)/Population(s) patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping Recommendation on Accelerated Approval Regulatory Action Recommended For the treatment of Duchenne muscular dystrophy (DMD) in Indication(s)/Population(s) patients who have a confirmed mutation of the DMD gene that (if applicable) is amenable to exon 45 skipping 1 Reference ID: 4751845 Clinical Review David Hosford, Xiang Ling, Thomas Biel, Ashutosh Rao NDA 213,026: casimersen for DMD amenable to exon 45 skipping Table of Contents Glossary ........................................................................................................................................11 1. Executive Summary...............................................................................................................14 1.1. Product Introduction......................................................................................................14 1.2. -

Stríbrná and Julius Lukes Hassan Hashimi, Lindsay Mcdonald, Eva
Bioenergetics: Trypanosome Letm1 Protein Is Essential for Mitochondrial Potassium Homeostasis Hassan Hashimi, Lindsay McDonald, Eva Stríbrná and Julius Lukes J. Biol. Chem. 2013, 288:26914-26925. doi: 10.1074/jbc.M113.495119 originally published online July 26, 2013 Access the most updated version of this article at doi: 10.1074/jbc.M113.495119 Find articles, minireviews, Reflections and Classics on similar topics on the JBC Affinity Sites. Alerts: • When this article is cited • When a correction for this article is posted Click here to choose from all of JBC's e-mail alerts Supplemental material: http://www.jbc.org/content/suppl/2013/07/26/M113.495119.DC1.html This article cites 60 references, 26 of which can be accessed free at http://www.jbc.org/content/288/37/26914.full.html#ref-list-1 Downloaded from http://www.jbc.org/ at Edinburgh University Library on September 13, 2013 THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 288, NO. 37, pp. 26914–26925, September 13, 2013 © 2013 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. Trypanosome Letm1 Protein Is Essential for Mitochondrial Potassium Homeostasis*□S Received for publication, June 19, 2013, and in revised form, July 23, 2013 Published, JBC Papers in Press, July 26, 2013, DOI 10.1074/jbc.M113.495119 Hassan Hashimi‡§1, Lindsay McDonald‡2, Eva Strˇíbrná‡, and Julius Lukesˇ‡§3 From the ‡Institute of Parasitology, Biology Centre, Czech Academy of Sciences and the §Faculty of Science, University of South Bohemia, 370 05 Cˇeské Budeˇjovice (Budweis), Czech Republic Background: Letm1 is a mitochondrial protein attributed disparate roles, including cation/proton antiport and translation. -

Loss of Frataxin Induces Iron Toxicity, Sphingolipid Synthesis, and Pdk1
1 Loss of Frataxin induces iron toxicity, sphingolipid synthesis, and 2 Pdk1/Mef2 activation, leading to neurodegeneration 3 4 Kuchuan Chen1, Guang Lin2, Nele A. Haelterman1, Tammy Szu-Yu Ho3, Tongchao Li1, Zhihong 5 Li2, Lita Duraine4, Brett H. Graham1, 2, Manish Jaiswal2, 4, Shinya Yamamoto1, 2, 5, Matthew N. 6 Rasband1, 3, and Hugo J. Bellen1, 2, 3, 4, 5, * 7 1Program in Developmental Biology, Baylor College of Medicine, Houston, TX 77030, USA 8 2Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA 9 3Department of Neuroscience, Baylor College of Medicine, Houston, TX 77030, USA 10 4Howard Hughes Medical Institute, Baylor College of Medicine, Houston, TX 77030, USA 11 5Jan and Dan Duncan Neurological Research Institute, Texas Children’s Hospital, Houston, TX 77030, 12 USA 13 14 Keywords: Friedreich ataxia; Frataxin; iron; mitochondria; sphingolipid; Pdk1; Mef2; lipid 15 droplets; photoreceptors; Drosophila 16 17 *Corresponding author: [email protected] 18 19 20 21 1 22 Abstract 23 Mutations in Frataxin (FXN) cause Friedreich’s ataxia (FRDA), a recessive 24 neurodegenerative disorder. Previous studies have proposed that loss of FXN causes 25 mitochondrial dysfunction, which triggers elevated reactive oxygen species (ROS) and leads to 26 the demise of neurons. Here we describe a ROS independent mechanism that contributes to 27 neurodegeneration in fly FXN mutants. We show that loss of frataxin homolog (fh) in Drosophila 28 leads to iron toxicity, which in turn induces sphingolipid synthesis and ectopically activates 3- 29 phosphoinositide dependent protein kinase-1 (Pdk1) and myocyte enhancer factor-2 (Mef2). 30 Dampening iron toxicity, inhibiting sphingolipid synthesis by Myriocin, or reducing Pdk1 or 31 Mef2 levels, all effectively suppress neurodegeneration in fh mutants.