Voiding Dysfunction Due to Underactive Detrusor: Can One Restore Function? W10, 15 October 2012 09:00 - 12:00
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

35-40 Institute of Normal Anatomy
35 Lymphology 28 (1995) 35-40 Institute of Normal Anatomy (PP,AT), Institute of Histology and General Embryology (CM), and Department of Human Pathology (RS), University of Pavia, Italy ABSTRACT light and electron microscopy (2,3). To clarify vesical lymph drainage, we now examined the After endoscopic transurethral biopsies of fine structure of small lymphatic vessels (SL V) normal human urinary bladder, an extensive and their distribution in the normal human network of small initial lymphatic vessels was urinary bladder. Particular attention was depicted by means of light and electron directed to the structure and composition of microscopy. Using light microscopy, lymphatic the connective matrix that surrounds the vessels were seen in the mucosa and lymphatic vessels in the different regions of submucosa and formed a complex network in the bladder wall. the detrusor muscular coat. These lymphatics were characterized by an irregular and MATERIALS AND METHODS attenuated wall and increased in number and size from the superficial to the deeper region of Ten male subjects (40 to 60 years of age) the bladder. Ultrastructurally, the lymphatic who had symptoms of bladder outlet wall was characterized by endothelial cells obstruction were utilized for this investigation. joined together end-to-end or by complicated Two endoscopic transurethral biopsies from interdigitations. Often intercellular channels the lateral wall of the normal urinary bladder and gaps between two contiguous endothelial were performed under a constant bladder cells were present. A broad network of elastic distention after introduction of physiological and collagen fibers joined the lymphatic saline at 50 cm H20 constant pressure. The endothelial wall to the neighboring connective biopsy trocar was regulated to obtain vesical tissue. -

Clinical and Functional Anatomy of the Urethral Sphincter
Review Article International Neurourology Journal Int Neurourol J 2012;16:102-106 http://dx.doi.org/10.5213/inj.2012.16.3.102 pISSN 2093-4777 · eISSN 2093-6931 INJ Clinical and Functional Anatomy of the Urethral Sphincter Junyang Jung, Hyo Kwang Ahn, Youngbuhm Huh Department of Anatomy, Kyung Hee University School of Medicine, Seoul, Korea Continence and micturition involve urethral closure. Especially, insufficient strength of the pelvic floor muscles including the urethral sphincter muscles causes urinary incontinence (UI). Thus, it is most important to understand the main mechanism causing UI and the relationship of UI with the urethral sphincter. Functionally and anatomically, the urethral sphincter is made up of the internal and the external sphincter. We highlight the basic and clinical anatomy of the internal and the external sphinc- ter and their clinical meaning. Understanding these relationships may provide a novel view in identifying the main mechanism causing UI and surgical techniques for UI. Keywords: Urethral sphincters; Pudendal nerve; Autonomic nervous system; Urinary incontinence; Urination INTRODUCTION tomical damage to the ligaments, facial support, and pelvic floor musculature, including the levator ani [8]. The pudendal nerve The urethral sphincter is crucial for the maintenance of urinary innervating the EUS is susceptible to injury during vaginal birth continence [1,2]. The urethral sphincter refers to one of the fol- because it travels between the sacrospinous and sacrotuberous lowing muscles [3]: 1) the internal urethral sphincter (IUS), ligaments [9]. In this article, we discuss the basic and clinical which consists of smooth muscle and is continuous with the anatomy of the urethral sphincter and the relationship between detrusor muscle and under involuntary control, and 2) the ex- the urethral sphincter and UI. -
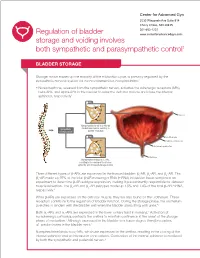
Regulation of Bladder Storage and Voiding Involves Both Sympathetic
Center for Advanced Gyn 5530 Wisconsin Ave Suite 914 Chevy Chase, MD 20815 301-652-1231 Regulation of bladder www.centerforadvancedgyn.com storage and voiding involves both sympathetic and parasympathetic control1 BLADDER STORAGE Storage, which makes up the majority of the micturition cycle, is primarily regulated by the sympathetic nervous system via the neurotransmitter, norepinephrine.2 • Norepinephrine, released from the sympathetic nerves, activates the adrenergic receptors (ARs), beta-ARs, and alpha-ARs in the bladder to relax the detrusor muscle and close the internal sphincter, respectively2 β-ARs Norepinephrine Ureter β-AR Norepinephrine binds to β-ARs on Sympathetic the detrusor muscle, resulting in nervous system bladder relaxation α-ARs Urothelium -AR α1 Detrusor muscle Internal sphincter Norepinephrine Urethra Norepinephrine binds to α1-ARs, resulting in the closing of the internal sphincter and increased storage of urine Three different types of b-ARs are expressed in the human bladder: b1-AR, b2-AR, and b3-AR. The b3-AR made up 97% of the total b-AR messenger RNA (mRNA) in bladder tissue samples in an experiment to determine b-AR subtype expression, making it predominantly responsible for detrusor muscle relaxation. The b1-AR and b2-AR subtypes made up 1.5% and 1.4% of the total b-AR mRNA, respectively.3 While b-ARs are expressed on the detrusor muscle, they are also found on the urothelium. These receptors contribute to the regulation of bladder function. During the storage phase, the urothelium stretches in tandem with the bladder wall when the bladder starts filling with urine.4,5 6 Both a1-ARs and a2-ARs are expressed in the lower urinary tract in humans. -
Bladder Retraining
James Paget University Hospitals NHS Foundation Trust Bladder retraining Patient Information How should my bladder work? Urine is made in the kidneys and travels down two tubes called the ureters to the bladder. The bladder is a storage organ that expands to store the urine. The urethra is the tube that runs from the bladder to the outside of your body, just in front of the vagina, or through the penis. A healthy bladder usually holds around 400ml-600ml (0.75–1 pint). The number of times you pass urine each day depends on a number of things, including how much fluid you drink. It may be normal to pass urine up to seven times a day and up to once during the night. If you are older (more than 70 years old) passing urine once or twice at night may be normal. 2 As the bladder starts to fill it sends a warning message to the brain. You normally feel the first warning when your bladder has about 200mls (1/3 of a pint) in it. If it is not convenient to go to the toilet at that time, your brain sends messages to your bladder telling it to relax. This allows you to continue with whatever you are doing at the time. The desire to empty your bladder will gradually become stronger and more difficult to put off or control. When you find an appropriate time to empty your bladder, you sit on the toilet (or probably stand up if you’re a man), relax your pelvic floor muscles and your bladder muscle (detrusor muscle) starts to contract, expelling the urine. -

The Female Pelvic Floor Fascia Anatomy: a Systematic Search and Review
life Systematic Review The Female Pelvic Floor Fascia Anatomy: A Systematic Search and Review Mélanie Roch 1 , Nathaly Gaudreault 1, Marie-Pierre Cyr 1, Gabriel Venne 2, Nathalie J. Bureau 3 and Mélanie Morin 1,* 1 Research Center of the Centre Hospitalier Universitaire de Sherbrooke, Faculty of Medicine and Health Sciences, School of Rehabilitation, Université de Sherbrooke, Sherbrooke, QC J1H 5N4, Canada; [email protected] (M.R.); [email protected] (N.G.); [email protected] (M.-P.C.) 2 Anatomy and Cell Biology, Faculty of Medicine and Health Sciences, McGill University, Montreal, QC H3A 0C7, Canada; [email protected] 3 Centre Hospitalier de l’Université de Montréal, Department of Radiology, Radio-Oncology, Nuclear Medicine, Faculty of Medicine, Université de Montréal, Montreal, QC H3T 1J4, Canada; [email protected] * Correspondence: [email protected] Abstract: The female pelvis is a complex anatomical region comprising the pelvic organs, muscles, neurovascular supplies, and fasciae. The anatomy of the pelvic floor and its fascial components are currently poorly described and misunderstood. This systematic search and review aimed to explore and summarize the current state of knowledge on the fascial anatomy of the pelvic floor in women. Methods: A systematic search was performed using Medline and Scopus databases. A synthesis of the findings with a critical appraisal was subsequently carried out. The risk of bias was assessed with the Anatomical Quality Assurance Tool. Results: A total of 39 articles, involving 1192 women, were included in the review. Although the perineal membrane, tendinous arch of pelvic fascia, pubourethral ligaments, rectovaginal fascia, and perineal body were the most frequently described structures, uncertainties were Citation: Roch, M.; Gaudreault, N.; identified in micro- and macro-anatomy. -

ANAT1551 Introductory Anatomy
Faculty of Medicine School of Medical Sciences ANAT1551 Introductory Anatomy Session 2 - 2013 Introductory Anatomy for Health & Exercise Science – Session 2, 2013 Contents Course convenor 2 Units of credit 2 Hours per week 2 What is anatomy? 2 Course aims 2 Student learning outcomes 2 Course relationships 3 Teaching rationale 4 Teaching strategies 4 Ethical behaviour and human remains 4 Anatomical terms 4 Assessment 5 Failure to sit a test 5 Resources for students 6 What is plagiarism? 6 Grievance procedures 7 Continual course improvement 7 Practical Class Attendance Requirement 7 Guidelines on extra-curricular activities affecting attendance 7 Health and Safety Rules for Students in the Dissecting Room 9 Course Lecture and Practical Class Schedule 12 Week 1: General Anatomy and Skeletal System 1 13 Week 2: Skeletal System 2 & Articular System 18 Week 3: Muscular System 1 22 Week 4: Muscular System 2 and Spinal Cord 26 Week 5: Spinal Nerves and the Autonomic Nervous System 29 Week 6: Revision Lab (9am-12pm Thursday 05/09/2013) 32 Spot Test No.1 (11am-12pm or 12-1pm Friday 06/09/2013) 32 Week 7: Brain and Cranial Nerves 33 Week 8: Eye and Ear 36 Week 9: Cardiovascular System 40 Week 10: Respiratory System 44 Week 11: Digestive System 47 Week 12: Urinary and Reproductive Systems 51 Week 13: Revision Lab (9am-12pm Thursday 31/10/2013) 54 Spot Test No.2 (11am-12pm or 12-1pm Friday 01/11/2013) 54 Cover illustration: One of the Vesalius plates (drawn by Calcar). From: Hixson, J. 1966. The History of the Human Body. -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -
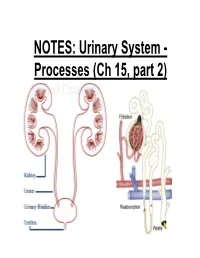
NOTES: Urinary System
NOTES: Urinary System - Processes (Ch 15, part 2) *Recall: the primary function of the urinary system is to filter the blood of ions and nitrogenous wastes; when combined with water, these wastes make up URINE. • Kidneys receive about 20-25% of total cardiac output – ~1200mL of blood goes through the kidneys per minute Blood Supply to Kidneys • Descending aorta Renal artery interlobar arcuate cortical afferent arterioles • The afferent arterioles deliver blood to nephrons NEPHRONS • NEPHRONS: the functional units of the kidneys -each kidney contains about a million nephrons! Parts of a NEPHRON: • GLOMERULUS: tangled cluster of blood capillaries • GLOMERULAR CAPSULE (a.k.a. Bowman’s capsule): thin-walled structure surrounding glomerulus Parts of a NEPHRON: • PROXIMAL CONVOLUTED TUBULE • LOOP OF HENLE -descending limb -ascending limb • DISTAL CONVOLUTED TUBULE Parts of a NEPHRON: • COLLECTING DUCT (where distal tubules from several nephrons converge and drain into; from here, urine empties into the RENAL PELVIS) Blood Supply of a Nephron: -blood is brought to a nephron from an afferent arteriole; -from here, it is passed to an efferent arteriole; -this gives rise to a system of peritubular capillaries that surround the renal tubules URINE FORMATION *nephrons remove wastes from blood and regulate water and electrolyte concentrations. URINE IS THE END PRODUCT! Three Organic Wastes Products of Urine 1) Urea: most abundant, from breakdown of amino acids 2) Creatinine: generated in skeletal muscle when creatine phosphate is broken down (creatine -

Bladder Dysfunction in Multiple Sclerosis
A RESOURCE FOR HEALTHCARE PROFESSIONALS BLADDER DYSFUNCTION IN MULTIPLE SCLEROSIS Rebecca Lavelle, MD Nancy J. Holland, EdD, RN Nancy C. Reitman, MA, RN Effective diagnosis and management of bladder dysfunction makes it possible for people with multiple sclerosis (MS) to continue their recreational and work activities with comfort, dignity and confidence. It is important to note that: • Bladder dysfunction occurs in approximately 80% of people living with MS, in people with minimal symptoms and those with major impairments. • Bladder symptoms, including frequency, urgency and incontinence, may negatively impact social and vocational activities. • Bladder symptoms are often mismanaged, precipitating such problems as acute urinary retention, damage to the detrusor (primary bladder muscle) and urinary tract infections (UTIs). NORMAL BLADDER FUNCTION The bladder wall consists of three main layers: the mucosa, submucosa, and detrusor muscle. The detrusor, a thick layer of smooth muscle, expands to store urine and contracts to expel urine. Storage and emptying of the bladder are regulated by the internal and external urethral sphincters. Sphincters are normally in a closed position, needing stimulation to open. Continence depends on sphincter–detrusor coordination. When approximately 250 to 300 cc of urine fill the bladder, the internal pressure activates stretch receptors in the bladder wall. The stretch receptors signal the nervous system, small contractile waves occur in the detrusor muscle, and the internal urethral sphincter automatically relaxes. The external sphincter is consciously tightened and the urge to urinate becomes apparent. Voluntary voiding occurs when two actions occur simultaneously: the detrusor muscle contracts to expel the urine and the external sphincter relaxes and opens to allow the urine to pass freely into the urethra and out of the body. -

Ta2, Part Iii
TERMINOLOGIA ANATOMICA Second Edition (2.06) International Anatomical Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TA2, PART III Contents: Systemata visceralia Visceral systems Caput V: Systema digestorium Chapter 5: Digestive system Caput VI: Systema respiratorium Chapter 6: Respiratory system Caput VII: Cavitas thoracis Chapter 7: Thoracic cavity Caput VIII: Systema urinarium Chapter 8: Urinary system Caput IX: Systemata genitalia Chapter 9: Genital systems Caput X: Cavitas abdominopelvica Chapter 10: Abdominopelvic cavity Bibliographic Reference Citation: FIPAT. Terminologia Anatomica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, 2019 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Anatomica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: SYSTEMA DIGESTORIUM Chapter 5: DIGESTIVE SYSTEM Latin term Latin synonym UK English US English English synonym Other 2772 Systemata visceralia Visceral systems Visceral systems Splanchnologia 2773 Systema digestorium Systema alimentarium Digestive system Digestive system Alimentary system Apparatus digestorius; Gastrointestinal system 2774 Stoma Ostium orale; Os Mouth Mouth 2775 Labia oris Lips Lips See Anatomia generalis (Ch. -

Introduction to the Urinary System
Tips for Success 1. Show up 2. Participate in lab 3. Show up 4. Turn in assignments (completed, refer to #6) 5. Show up 6. Communicate with me, e-mail is best 7. Show up Every point counts! Business Homework due in lab Label the handout provided today Front and back Use your book! There should be at least 18 items labeled Part 1 Urinary System Wastes Gases versus fluids Urinary system Dispose of water soluble wastes Electrolyte regulation Acid-base regulation Urinary System Other functions Kidneys Renin Erythropoietin Vitamin D activation Nitrogenous Wastes Urine is about 95% water Second largest component is urea Urea derived from breakdown of amino acids Nitrogenous Wastes TOXIC! + 1. Dietary amino acids → NH2 removed → NH2 + H → NH3 500 ml of urine removes only 1 gram of nitrogen as ammonia 2. Ammonia can be converted to urea Requires energy 50 ml of urine removes 1 gram of nitrogen as urea 3. Ammonia can be converted to uric acid Requires lots of energy 10 ml of urine removes 1 gram of nitrogen as uric acid Urinary System Organs Kidneys Major excretory organs Urinary bladder Temporary storage reservoir for urine Ureters Transport urine from the kidneys to the bladder Urethra Transports urine out of the body Hepatic veins (cut) Esophagus (cut) Inferior vena cava Renal artery Adrenal gland Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female reproductive Urinary system) bladder Urethra Figure 25.1 Kidney: Urinary System page 6 Anterior Inferior vena cava Peritoneal -

The Urinary System
9. THE URINARY SYSTEM The urinary system comprises of an excretory part (the kidneys) and a collecting part (the renal calyces and pelvis, the ureter and the bladder) which ends up at the urethra, by which the urine is expelled during voiding (urination). The main function of the urinary system is to filter the blood, eliminate useless and excessive substances and regulate the water, electrolyte and acid-base equilibrium of the body. The kidneys, however, are more than “filters”. They also actively control important functions of the human body, such as blood pressure regulation, erythropoeisis (production of red blood cells by the bone marrow) and Vitamin D activation. The urinary tract is similar in both males and females, only down to the level of the urethra. In the male the urethra is longer, as it has to travel through the prostate and penis and is also shared by the genital system, where it conveys semen during ejaculation. The kidneys The kidneys are two bean-shaped organs, located on either side of the vertebral column in the abdominal cavity, behind the peritoneum (in the retroperitoneal space). They lie at the level of the 12th thoracic to the 3d lumbar vertebrae. The right kidney is situated lower (approx. ½ vertebra) than the left one, because of the position of the liver. The kidneys are approximately 12 cm long, 6-7 cm wide and 2.5-3 cm thick. At their concave, medial surface lies the hilum, the area where the renal pelvis and ureter exits and the renal vessels (artery and vein) enter and exit, respectively.