Supporting Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
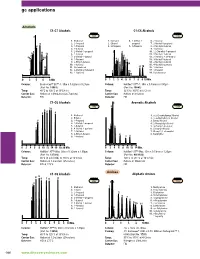
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing A-Cyano Or A-Ester Functional Group from Baylis-Hillman Adducts
Notes Bull. Korean Chem. Soc. 2004, Vol. 25, No. 3 413 A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing a-Cyano or a-Ester Functional Group from Baylis-Hillman Adducts Ka Young Lee, Saravanan GowriSankar, and Jae Nyoung Kim Department of Chemistry and Institute of Basic Science, Chonnam National University, Gwangju 500-757, Korea Received December 18, 2003 Key Words : Cinnamyl alcohols, Baylis-Hillman adducts, Sulfuric acid, Acetic anhydride Cinnamyl alcohols bearing ester, acid, or nitrile functional Hillman alcohol into the primary acetate or eventually to group at the 2-position are very useful synthons for the primary alcohol (Scheme 1 and Table 1). synthesis of various biologically important compounds.1 The reaction of Baylis-Hillman adducts with acetic Synthesis of cinnamyl alcohol derivatives from the Baylis- anhydride (1.5 equiv.) in the presence of sulfuric acid (10 Hillman adducts has been reported by us and other groups.1-4 mol%) gave the corresponding rearranged cinnamyl acetates The conversion can be carried out directly by using 20% quantitatively in dichloromethane at room temperature aqueous sulfuric acid (for the Baylis-Hillman adducts within 30 min (Actually, the cinnamyl acetate derivatives derived from acrylonitrile)2 or trifluoroacetic acid (for the could be isolated in 92-95% yields.). In order to obtain the Baylis-Hillman adducts derived from acrylates).3 Recently, corresponding cinnamyl alcohols in a one-pot we tried some Basavaiah and coworkers have reported the two-step, one- reaction conditions for the next partial hydrolysis. Addition pot conversion method: TMSOTf-assisted conversion of of water to the reaction mixture (two-phase system) afforded Baylis-Hillman alcohol into the corresponding primary the hydrolyzed cinnamyl alcohols in trace amounts. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Tolu ................................................................ Myroxylon balsamum (L.) Harms. Turpentine ...................................................... Pinus palustris Mill. and other Pinus spp. which yield terpene oils exclusively. Valerian rhizome and roots ............................ Valeriana officinalis L. Veronica ......................................................... Veronica officinalis L .................................................. Do. Vervain, European ......................................... Verbena officinalis L ................................................... Do. Vetiver ............................................................ Vetiveria zizanioides Stapf ......................................... Do. Violet, Swiss ................................................... Viola calcarata L. Walnut husks (hulls), leaves, and green nuts Juglans nigra L. or J. regia L. Woodruff, sweet ............................................. Asperula odorata L ..................................................... In alcoholic beverages only Yarrow ............................................................ Achillea millefolium L .................................................. In beverages only; fin- ished beverage thujone free1 Yerba santa .................................................... Eriodictyon californicum (Hook, et Arn.) Torr. Yucca, Joshua-tree ........................................ Yucca brevifolia Engelm. Yucca, -

Nomination Background: Methyl Trans
' 7/?1 Methyl styryl ketone 122-57-6 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Nomination Source: NCI 2. Recommendations: -Comparative toxicity studies with methyl vinyl ketone -Metabolism -Carcinogenicity 3. Rationale/Remarks: -Potential for widespread human exposure -Nominated as a result of a class study of a,~-unsaturated ketones -Flavoring and fragrance additive -Natural product -Environmental pollutant -Mutagenic in Salmonella 4. Priority: -High for toxicity studies -Moderate for carcinogenicity s. Date of Nomination: July 1994 B. Interagency Committee for Chemical Evaluation and Coordination Review 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: 122-57-6 Methyl styryl ketone SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Number: 122-57-6 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl- (SCI, 9CI) Svnonvms and Trade Names: Acetocinnamone; benzalacetone; benzylideneacetone; methyl 2 phenylvinyl ketone; methyl styryl ketone; methyl ~-styryl ketone; 4 phenylbutenone; 4-phenyl-3-butene-2-one; 2-phenylvinyl methyl ketone; styryl methyl ketone; MSK FEMA Number: 2881 Related isomers CAS Registry Number: 937-53-1 Chemica] Abstracts Name: 3-Buten-2-one, 4-phenyl-, (Z)- (SCI, 9CI) Synonvms and Trade Names: cis -4-Phenyl-3-buten-2-one; cis -benzalacetone; cis benzylideneacetone; cis-methyl styryl ketone CAS Registry Number: 1896-62-4 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl-, (E)- (SCI, 9CI) Synonyms and Trade Names: trans-4-Phenyl-3-buten-2-one; trans-benzalacetone; trans benzylideneacetone; trans-methyl styryl ketone; TPBO Structure. Molecular Formula. and Molecular WeiKbt 0 II H•CHCCH3 Mol. wt.: 146.19 Chemical and Phvsical Properties (from Aldrich Chemical Co., 1993, 1994; Budavari, 1989) Description: White to yellow solid with a sweet, pungent, creamy, floral odor Prepared for NCI by Technical Resources, Inc. -

Functional Expression of a Novel Methanol-Stable Esterase From
Cai et al. BMC Biotechnology (2020) 20:36 https://doi.org/10.1186/s12896-020-00622-1 RESEARCH ARTICLE Open Access Functional expression of a novel methanol- stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent- free system Xianghai Cai1†, Lin Lin2,3†, Yaling Shen1, Wei Wei1* and Dong-zhi Wei1 Abstract Background: Esterases are widely distributed in nature and have important applications in medical, industrial and physiological. Recently, the increased demand for flavor esters has prompted the search of catalysts like lipases and esterases. Esterases from thermophiles also show thermal stability at elevated temperatures and have become enzymes of special interest in biotechnological applications. Although most of esterases catalyzed reactions are carried out in toxic and inflammable organic solvents, the solvent-free system owning many advantages such as low cost and easy downstream processing. Results: The gene estGSU753 from Geobacillus subterraneus DSM13552 was cloned, sequenced and overexpressed into Escherichia coli BL21 (DE3). The novel gene has an open reading frame of 753 bp and encodes 250-amino-acid esterase (EstGSU753). The sequence analysis showed that the protein contains a catalytic triad formed by Ser97, Asp196 and His226, and the Ser of the active site is located in the conserved motif Gly95-X-Ser97-X-Gly99 included in most esterases and lipases. The protein catalyzed the hydrolysis of pNP-esters of different acyl chain lengths, and the enzyme specific activity was 70 U/mg with the optimum substrate pNP-caprylate. The optimum pH and temperature of the recombinant enzyme were 8.0 and 60 °C respectively. -

Proceedings of the Indiana Academy of Science
The Reactions of Silicomolybdic Acid with Organic Compounds 1 John H. Billman, Donald B. Borders, and John A. Buehler, Indiana University, Hondo AFB, Texas, and Anderson College For many years the analytical and inorganic chemists have been seeking specific reagents to identify metallic ions. At the same time, the organic chemist has been interested in finding compounds that would be useful for spotting different types of functional groups commonly en- countered in organic compounds. A typical example of such a reagent is ferric chloride, which has found wide use with phenols. For several years we have been testing various compounds in an effort to find reagents that would be specific for metallic ions as well as organic functional groups. In the course of our investigation it was found that silicomolybdic acid (1), H 4 SiMo 12 O 40 .xH 2 O, would produce a dark blue mixture in the presence of aliphatic aldehydes, but would not give the same test with aromatic aldehydes. Further investigation indi- cated that only aldehydes that contain a hydrogen atom on the alpha carbon atom would give a positive test. On the basis of this work a more thorough study was undertaken of the reaction of silicomolybdic acid with various organic compounds containing different functional groups. Altogether the reagent has been tried with a one-hundred and forty-five organic compounds. Table 1 shows the aldehydes that have been tested. TABLE I Aldehydes — Formaldehyde — Benzaldehyde + Acetaldehyde — m-Nitrobenzaldehyde -f Phenylacetaldehyde — p-Nitrobenzaldehyde -

Alcohol Dehydrogenase and Cinnamoyl-Coa Reductase (Monolignol͞genetic Modification͞antisense Rna͞coniferyl Alcohol͞feruloyl-Coa)
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 12803–12808, October 1998 Biochemistry NMR characterization of altered lignins extracted from tobacco plants down-regulated for lignification enzymes cinnamyl- alcohol dehydrogenase and cinnamoyl-CoA reductase (monolignolygenetic modificationyantisense RNAyconiferyl alcoholyferuloyl-CoA) JOHN RALPH*†‡,RONALD D. HATFIELD*, JOE¨L PIQUEMAL§,NABILA YAHIAOUI§,MICHEL PEAN¶, i CATHERINE LAPIERRE , AND ALAIN M. BOUDET§ *United States Dairy Forage Research Center, United States Department of Agriculture, Agricultural Research Service, Madison, WI 53706-1108; †Department of Forestry, University of Wisconsin, Madison, WI 53706-1598; §Unite´Mixte de Recherche, Centre National de la Recherche Scientifique 5546, Centre de Biologie et Physiologie Ve´ge´tale, Universite´Paul Sabatier Baˆt.4R1, 118 route de Narbonne, F-31062 Toulouse Cedex, France; ¶Commissariat a`l’Energie Atomique, De´partementd’Ecophysiologie Ve´ge´tale et Microbiologie, Cadarache, Baˆt.177, F-13108 Saint Paul lez Durance Cedex, France; and iInstitut National de la Recherche Agronomique, Laboratoire de Chimie Biologique, F-78850 Thiverval-Grignon, France Communicated by Ron Sederoff, North Carolina State University, Raleigh, NC, July 29, 1998 (received for review May 13, 1998) OHO OS OH OH ABSTRACT Homologous antisense constructs were CoA used to down-regulate tobacco cinnamyl-alcohol dehydro- CCL' CCR' CAD' p-Coumaryl genase (CAD; EC 1.1.1.195) and cinnamoyl-CoA reductase alcohol (CCR; EC 1.2.1.44) activities in the lignin monomer biosyn- OH OH OH OH 1) HYD1' 1) HYD2' 1) HYD3' 1) HYD4' thetic pathway. CCR converts activated cinnamic acids 2) OMT1' 2) OMT2' 2) OMT3' 2) OMT4' OHO OS OH OH (hydroxycinnamoyl–SCoAs) to cinnamaldehydes; cinnama- CoA CCR CAD ldehydes are then reduced to cinnamyl alcohols by CAD. -

X-ADH Is the Sole Alcohol Dehydrogenase Isozyme of Mammalian Brains: Implications and Inferences (Class III Alcohol Dehydrogenase/Tissue-Specific Enzyme) THOMAS B
Proc. Natl. Acad. Sci. USA Vol. 82, pp. 8369-8373, December 1985 Biochemistry X-ADH is the sole alcohol dehydrogenase isozyme of mammalian brains: Implications and inferences (class III alcohol dehydrogenase/tissue-specific enzyme) THOMAS B. BEISSWENGER, BARTON HOLMQUIST, AND BERT L. VALLEE* Center for Biochemical and Biophysical Sciences and Medicine, Harvard Medical School, 250 Longwood Avenue, Boston, MA 02115 Contributed by Bert L. Vallee, August 14, 1985 ABSTRACT Class m (X) is the only alcohol dehydrog- occurrence and function of ADH in that tissue has always enase (ADH) in human, equine, bovine, simian, canine, and been of particular interest, but efforts to uncover facts rodent brain and is the first to be identified, purified, and relevant to ethanol have been singularly disappointing. Most characterized from the brain of humans or other vertebrates. of those studies antedate the awareness of the remarkable Like the corresponding isozymes from human placenta and number and diversity of structure and specificity of ADH liver, the X-ADH isozymes purified from mammalian brain are isozymes (4), first noted much earlier (5). neither inhibited by nor do they bind to immobilized pyrazole, Experimental work failed to reveal any significant ADH and they oxidize ethanol only very poorly (Km > 2.5 M). activity in brain (6). The level ofethanol oxidation in rat brain Indeed, it would be incorrect to classify them as "ethanol is so vanishingly low that only assays of exceptionally high dehydrogenases." They contain 4 g.atom of zinc/mol, bind 2 sensitivity could detect it (7). A number of contemporaneous moles of NAD, and readily oxidize long-chain aliphatic and studies employing [14C]ethanol concluded that the perfused aromatic primary alcohols. -

RIFM Fragrance Ingredient Safety Assessment, Α-Amylcinnamyl Alcohol, CAS T Registry Number 101-85-9 A.M
Food and Chemical Toxicology 134 (2019) 110712 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short review RIFM fragrance ingredient safety assessment, α-amylcinnamyl alcohol, CAS T Registry Number 101-85-9 A.M. Apia, D. Belsitob, S. Bisertaa, D. Botelhoa, M. Bruzec, G.A. Burton Jr.d, J. Buschmanne, M.A. Cancellieria, M.L. Daglif, M. Datea, W. Dekantg, C. Deodhara, A.D. Fryerh, S. Gadhiaa, L. Jonesa, K. Joshia, A. Lapczynskia, M. Lavellea, D.C. Liebleri, M. Naa, D. O'Briena, A. Patela, T.M. Penningj, G. Ritaccoa, F. Rodriguez-Roperoa, J. Rominea, N. Sadekara, D. Salvitoa, ∗ T.W. Schultzk, F. Siddiqia, I.G. Sipesl, G. Sullivana, , Y. Thakkara, Y. Tokuram, S. Tsanga a Research Institute for Fragrance Materials, Inc, 50 Tice Boulevard, Woodcliff Lake, NJ, 07677, USA b Member Expert Panel, Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave, New York, NY, 10032, USA c Member Expert Panel, Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE, 20502, Sweden d Member Expert Panel, School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St, Ann Arbor, MI, 58109, USA e Member Expert Panel, Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625, Hannover, Germany f Member Expert Panel, University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. Prof. dr. Orlando Marques de Paiva, 87, Sao Paulo, CEP 05508-900, Brazil g Member Expert Panel, University of Wuerzburg, Department of Toxicology, Versbacher Str. -

Safety Data Sheets
SAFETY DATA SHEETS According to Globally Harmonized System of Classification and Labelling of Chemicals (GHS) - Sixth revised edition Version: 1.0 Creation Date: Feb. 6, 2018 Revision Date: Feb. 6, 2018 1. Identification 1.1 GHS Product identifier Product name Cinnamyl alcohol 1.2 Other means of identification Product number A506174 Other names 3-Phenyl 1.3 Recommended use of the chemical and restrictions on use Identified uses Used for research and development only.Food additives -> Flavoring Agents Uses advised against no data available 1.4 Supplier's details Company Sangon Biotech (Shanghai) Co., Ltd. Address 698 Xiangmin Road, Songjiang, Shanghai 201611, China Telephone +86-400-821-0268 / +86-800-820-1016 Fax +86-400-821-0268 to 9 1.5 Emergency phone number Emergency phone +86-21-57072055 number Service hours Monday to Friday, 9am-5pm (Standard time zone: UTC/GMT +8 hours). 2. Hazard identification 2.1 Classification of the substance or mixture Skin irritation, Category 2 Eye irritation, Category 2 2.2 GHS label elements, including precautionary statements Pictogram(s) Signal word Warning Cinnamyl alcohol Page 1 of 8 Hazard statement(s) H315 Causes skin irritation H319 Causes serious eye irritation Precautionary statement(s) Prevention P264 Wash ... thoroughly after handling. P280 Wear protective gloves/protective clothing/eye protection/face protection. Response P302+P352 IF ON SKIN: Wash with plenty of water/... P321 Specific treatment (see ... on this label). P332+P313 If skin irritation occurs: Get medical advice/attention. P362+P364 Take off contaminated clothing and wash it before reuse. P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. -

Arabidopsis Thaliana Defense-Related Protein ELI3 Is an Aromatic Alcohol:NADP؉ Oxidoreductase (Benzyl Alcohol Dehydrogenase͞disease Resistance͞fungal Elicitor)
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 14199–14203, November 1996 Plant Biology Arabidopsis thaliana defense-related protein ELI3 is an aromatic alcohol:NADP1 oxidoreductase (benzyl alcohol dehydrogenaseydisease resistanceyfungal elicitor) IMRE E. SOMSSICH*, PETRA WERNERT,SIEGRID KIEDROWSKI†, AND KLAUS HAHLBROCK Max Planck Institut fu¨r Zu¨chtungsforschung, Department of Biochemistry, D-50829 Ko¨ln, Germany Contributed by Klaus Hahlbrock, September 12, 1996 ABSTRACT We expressed a cDNA encoding the Arabi- presence of the RPMI resistance gene in A. thaliana (7). The dopsis thaliana defense-related protein ELI3-2 in Escherichia RPM1 locus confers resistance to Pseudomonas syringae strains coli to determine its biochemical function. Based on a protein carrying the corresponding avirulence (avr) gene avrRpm1 (8). database search, this protein was recently predicted to be a The ELI3 cDNAs from parsley and A. thaliana share 67% mannitol dehydrogenase [Williamson, J. D., Stoop, J. M. H., nucleotide and 70% deduced amino acid sequence identity (7). Massel, M. O., Conkling, M. A. & Pharr, D. M. (1995) Proc. At the time of their isolation, no related sequences were found Natl. Acad. Sci. USA 92, 7148–7152]. Studies on the substrate in the various data bases. Meanwhile, several plant cinnamyl- specificity now revealed that ELI3-2 is an aromatic alcohol: alcohol dehydrogenases (CAD) with similarity to the deduced NADP1 oxidoreductase (benzyl alcohol dehydrogenase). The ELI3 proteins have been reported (9). Based on this similarity, enzyme showed a strong preference for various aromatic it was possible that the eli3 gene encodes a CAD. Recently, aldehydes as opposed to the corresponding alcohols. Highest however, Williamson et al. -

Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde Over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles
catalysts Article Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles Zahoor Iqbal 1,* , Muhammad Sufaid Khan 1,*, Rozina Khattak 2, Tausif Iqbal 3, Ivar Zekker 4, Muhammad Zahoor 5 , Helal F. Hetta 6 , Gaber El-Saber Batiha 7 and Eida M. Alshammari 8 1 Department of Chemistry, University of Malakand, Chakdara 18800, Pakistan 2 Department of Chemistry, Shaheed Benazir Bhutto Women University, Peshawar 25000, Pakistan; [email protected] 3 Center for Computational Materials Science, University of Malakand, Chakdara 18800, Pakistan; [email protected] 4 Institute of Chemistry, University of Tartu, 14a Ravila St., 50411 Tartu, Estonia; [email protected] 5 Department of Biochemistry, University of Malakand, Chakdara 18800, Pakistan; [email protected] 6 Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH 45267-0595, USA; [email protected] 7 Department of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Damanhour 22511, El-Beheira, Egypt; [email protected] 8 Department of Chemistry, College of Sciences, University of Ha’il, Ha’il 81451, Saudi Arabia; Citation: Iqbal, Z.; Khan, M.S.; [email protected] Khattak, R.; Iqbal, T.; Zekker, I.; * Correspondence: [email protected] (Z.I.); [email protected] (M.S.K.) Zahoor, M.; Hetta, H.F.; El-Saber Batiha, G.; Alshammari, E.M. Abstract: The selective oxidation of alcohols to aldehydes has attracted a lot of attention because of Selective Oxidation of Cinnamyl its potential use in agrochemicals, fragrances, and fine chemicals. However, due to homogenous Alcohol to Cinnamaldehyde over catalysis, low yield, low selectivity, and hazardous oxidants, traditional approaches have lost their Functionalized Multi-Walled Carbon efficiency.