Aldrich Alcohols and Phenols
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Inositol Safety: Clinical Evidences
European Review for Medical and Pharmacological Sciences 2011; 15: 931-936 Inositol safety: clinical evidences G. CARLOMAGNO, V. UNFER AGUNCO Obstetrics & Gynecology Center, Rome (Italy) Abstract. – Myo-inositol is a six carbon ent required by the human cells for the growth cyclitol that contains five equatorial and one axi- and survival in the culture. In humans and other al hydroxyl groups. Myo-inositol has been classi- species, Myo-inositol can be converted to either fied as an insulin sensitizing agent and it is L- or D-chiro-inositol by epimerases. Early stud- commonly used in the treatment of the Polycys- tic Ovary Syndrome (PCOS). However, despite ies showed that inositol urinary clearance was al- its wide clinical use, there is still scarce informa- tered in type 2 diabetes patients, the next step tion on the myo-inositol safety and/or side ef- was to link impaired inositol clearance with in- fects. The aim of the present review was to sum- sulin resistance (for a review see1). Because of marize and discuss available data on the myo-in- these properties, inositol have been classified as ositol safety both in non-clinical and clinical set- “insulin sensitizing agent”2. tings. The main outcome was that only the highest In the recent years, inositol has found more dose of myo-inositol (12 g/day) induced mild and more space in the reproductive clinical prac- gastrointestinal side effects such as nausea, fla- tice3-6. Indeed, since the main therapy for Poly- tus and diarrhea. The severity of side effects did cystic Ovary Syndrome (PCOS) is the use of in- not increase with the dosage. -
Revisions to USP 31–NF 26, Second Supplement
Revisions to USP 31–NF 26, Second Supplement (published May 2008) Published April 2008 General Chapters Monographs:A–C D–N O–S T–Z Monograph Title Section Head Scientific Liaison DANTROLENE SODIUM Dissolution <711> CAPSULES PF 33(4) Pg. 645 DEHYDROACETIC ACID PF 33(4) Title Pg. 703 DEHYDROACETIC ACID PF 33(4) Chemical Info Pg. 703 DEHYDROACETIC ACID PF 33(4) Definition Pg. 703 DEHYDROACETIC ACID PF 33(4) Packaging and storage Pg. 703 DEHYDROACETIC ACID PF 33(4) USP Reference standards <11> Pg. 703 DEHYDROACETIC ACID PF 33(4) Identification Pg. 703 DEHYDROACETIC ACID PF 33(4) Heavy Metals, Method II <231> Pg. 703 DEHYDROACETIC ACID PF 33(4) Loss on drying <731> Pg. 703 DEHYDROACETIC ACID PF 33(4) Melting range, Class I <741> Pg. 703 DEHYDROACETIC ACID PF 33(4) Residue on ignition <281> Pg. 703 DEHYDROACETIC ACID PF 33(4) Assay Pg. 703 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Title 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Definition 784 file:///C|/Documents%20and%20Settings/rwt/Desktop/revisions/usp31nf26secondSupplement03.html[4/26/2011 1:18:07 PM] DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Packaging and storage 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Labeling 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. USP Reference stadards 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Identification 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Uniformity of dosage units 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. Loss on drying 784 DIDANOSINE TABLETS FOR ORAL SUSPENSION PF 32(3) Pg. -

GRAS Notice 789 for Erythritol
GRAS Notice (GRN) No. 789 https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory. Toi• Strategies ~~~~G~~[)) JUN 7 20'8 Innovative solutions Sound science OFFICE OF FOOD ADDITIVE SAFE1Y June 5, 2018 Dr. Dennis Keefe Director, Division of Biotechnology and GRAS Notice Review Office of Food Additive Safety (HFS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Paint Branch Parkway College Park, MD 20740-3835 Subject: GRAS Notification - Erythritol Dear Dr. Keefe: On behalf of Cargill, Incorporated, ToxStrategies, Inc. (its agent) is submitting, for FDA review, a copy of the GRAS notification as required. The enclosed document provides notice of a claim that the food ingredient, erythritol, described in the enclosed notification is exempt from the premarket approval requirement of the Federal Food, Drug, and Cosmetic Act because it has been determined to be generally recognized as safe (GRAS), based on scientific procedures, for addition to food. If you have any questions or require additional information, please do not hesitate to contact me at 630-352-0303, or [email protected]. Sincerely, (b) (6) Donald F. Schmitt, M.P.H. Senior Managing Scientist ToxStrategies, Inc., 931 W. 75th St. , Suite 137, PMB 263, Naperville, IL 60565 1 Office (630) 352-0303 • www.toxstrategies.com GRAS Determination of Erythritol for Use in Human Food JUNES,2018 Innovative solutions s ,..,.,',--.r-.r--.r--. OFFICE OF FOOD ADDITIVE SAFE1Y GRAS Determination of Erythritol for Use in Human Food SUBMITTED BY: Cargill, Incorporated 15407 McGinty Road West Wayzata, MN 55391 SUBMITTED TO: U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food Additive Safety HFS-200 5100 Paint Branch Parkway College Park MD 20740-3835 CONTACT FOR TECHNICAL OR OTIIER INFORMATION Donald F. -

“Polyols: a Primer for Dietetic Professionals” Is a Self-Study
1 “Polyols: A primer for dietetic professionals” is a self-study module produced by the Calorie Control Council, an accredited provider of continuing professional education (CPE) for dietetic professionals by the Commission on Dietetic Registration. It provides one hour of level 1 CPE credit for dietetic professionals. The full text of the module is in the notes section of each page, and is accompanied by summary points and/or visuals in the box at the top of the page. Directions for obtaining CPE are provided at the end of the module. 2 After completing this module, dietetic professionals will be able to: • Define polyols. • Identify the various types of polyols found in foods. • Understand the uses and health effects of polyols in foods. • Counsel clients on how to incorporate polyols into an overall healthful eating pattern. 3 4 Polyols are carbohydrates that are hydrogenated, meaning that a hydroxyl group replaces the aldehyde or ketone group found on sugars. Hydrogenated monosaccharides include erythritol, xylitol, sorbitol, and mannitol. Hydrogenated disaccharides include lactitol, isomalt, and maltitol. And hydrogenated starch hydrolysates (HSH), or polyglycitols (a wide range of corn syrups and maltodextrins), are formed from polysaccharides (Grabitske and Slavin 2008). 5 Nearly 54 percent of Americans are trying to lose weight, more than ever before. Increasingly, they are turning toward no- and low-sugar, and reduced calorie, foods and beverages to help them achieve their weight loss goals (78% of Americans who are trying to lose weight) (CCC 2010). Polyols, found in many of these foods, are becoming a subject of more interest. 6 They are incompletely digested , therefore are sometimes referred to as “low- digestible carbohydrates.” Polyols are not calorie free, as there is some degree of digestion and absorption of the carbohydrate. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -
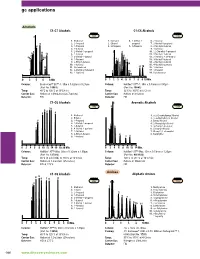
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Borneol - Camphor - Isoborneol
EXP. 35 A & B OXIDATION-REDUCTION SCHEME: BORNEOL - CAMPHOR - ISOBORNEOL LEARNING OBJECTIVES: To illustrate the concepts of oxidation and reduction in organic chemistry, to illustrate the stereochemical effects of these reactions in certain systems, to use IR spectroscopy to characterize diastereomers and monitor reactions. QUIZ PREPARATION: Recitation notes and readings assigned in the syllabus. OXIDATION AND REDUCTION IN ORGANIC CHEMISTRY The concepts of oxidation and reduction in chemistry are fundamentally related to the loss and gain of electrons, respectively. For a specific atom, oxidation brings about an increase in the oxidation number, whereas reduction does the opposite. This is of course true in organic reactions as well, but it is frequently less clear where the gain or loss of electrons is taking place. For this reason, the concepts of oxidation and reduction are addressed from a different perspective in organic chemistry. Since carbon is the most important element in organic chemistry, we can define oxidation as a process whereby carbon gains bonds to more electronegative atoms. These can be any atoms more electronegative than carbon, such as chlorine or sulphur, but the most commonly seen in organic oxidations is oxygen. The more bonds to oxygen, the higher the oxidation state of the carbon involved. Examples of oxidation reactions are those that convert alkanes into alcohols, or alcohols into carbonyl compounds. The functional groups that contain the most highly oxidized form of carbon in organic compounds are the carboxylic acids and their derivatives. Reduction is the opposite of oxidation. We can define reduction as a process whereby carbon adds bonds to less electronegative atoms. -

United States Patent (19) 11 Patent Number: 6,037,477 Ishii Et Al
USOO6037477A United States Patent (19) 11 Patent Number: 6,037,477 Ishii et al. (45) Date of Patent: Mar. 14, 2000 54) OXIDATION PROCESS OF ETHERS 838909 2/1996 Japan. 75 Inventors: Yasutaka Ishii, 19-21, OTHER PUBLICATIONS Besshohonmachi, Takatsuki-shi, Osaka Ishii et al., J. Org. Chem., vol. 61, pp. 4520-4526 (1996). 569-1112, Tatsuya Nakano, Himeji, Yoshino et al., J. Org. Chem., vol. 62, No. 20, pp. both of Japan 6810–6813 (Oct. 1997). Takeno et al., Aerobic Oxidation by Using N-hydroxyph 73 Assignees: Daicel Chemical Industries, Ltd.; thalimide, 67th Spring Annual Meeting of Chemical Society Yasutaka Ishii, both of Osaka, Japan of Japan, Lecture Draft II, Dec. 1994. 21 Appl. No.: 09/074,604 Primary Examiner Johann Richter ASSistant Examiner-Dominic Keating 22 Filed: May 8, 1998 Attorney, Agent, or Firm-Birch, Stewart, Kolasch & Birch, 30 Foreign Application Priority Data LLP May 13, 1997 JP Japan .................................... 9-122526 57 ABSTRACT 51) Int. Cl." .................. C07D 207/404; CO7D 207/448; An ether is oxidized with oxygen under an oxidation catalyst CO7D 487/06; CO7D 207/444 comprising an imide compound (Such as 52 U.S. Cl. .......................... 548/545; 548/548; 548/549; N-hydroxyphthalimide) or the imide compound and a 548/453 co-catalyst to produce the corresponding chain or cyclic 58 Field of Search ..................................... 548/545, 548, ester or anhydride. The co-catalyst may be a transition metal 548/549 compound. The above proceSS provides a process for oxi dizing an ether by oxygen efficiently to produce the corre 56) References Cited sponding oxide (Such as an ester, an hydride) with high conversion and Selectivity. -

Butenes Separation, Supp. A
PROCESS ECONOMICS PROGRAM SRI INTERNATIONAL Menlo Park, California 94025 Abstract Process Economics Program Report No. 71A BUTTLENES (October 1982) Demand is fast increasing for lsobutylene, especially that used in manufacturing methyl tertiary-butyl ether, and for high purity butene-1 to use as a copolymer in linear low density polyethylene. Because of their wide availability, mixed butane-butylene streams from oleflns plants and petroleum refineries are being increasingly fed to plants to separate butylenes for use in chemicals. This first supplement to Report No. 71 updates demand projections, production capacities, and separation techniques for high purity butene-1 and lsobutylene. The processes that are now available for separating and purifying both butene-1 and lsobutylene from mixed butyl- ene streams are evaluated and compared. Other procedures for obtaining butylenes, such as dehydrogenatlon, lsomerleatlon, and disproportion&ion, are not updated in this report. PEP’81 JLC Report No. 71A - BUTYLENES SUPPLEMENT A by JOHN L. CHADWICK I I October 1982 f-F0 0 A private report by the m PROCESS ECONOMICS PROGRAM 0 Menlo Park, California 94025 0 For detailed marketing data and information, the reader is referred to one of the SRI programs specializing in marketing research. The CHEMICALECONOMICS HANDBOOK Program covers most major chemicals and chemical products produced in the United States and the WORLDPETROCHEMICALS Program covers major hkdrocarbons and their derivatives on a worldwide basis. In addition, the SRI DIRECTORYOF CHEMICALPRODUCERS services provide detailed lists of chemical producers by company, prod- uct, and plant for the United States and Western Europe. ii CONTENTS 1 INTRODUCTION . 1 2 SUMMARY . -

The Alcohol Textbook 4Th Edition
TTHEHE AALCOHOLLCOHOL TEXTBOOKEXTBOOK T TH 44TH EEDITIONDITION A reference for the beverage, fuel and industrial alcohol industries Edited by KA Jacques, TP Lyons and DR Kelsall Foreword iii The Alcohol Textbook 4th Edition A reference for the beverage, fuel and industrial alcohol industries K.A. Jacques, PhD T.P. Lyons, PhD D.R. Kelsall iv T.P. Lyons Nottingham University Press Manor Farm, Main Street, Thrumpton Nottingham, NG11 0AX, United Kingdom NOTTINGHAM Published by Nottingham University Press (2nd Edition) 1995 Third edition published 1999 Fourth edition published 2003 © Alltech Inc 2003 All rights reserved. No part of this publication may be reproduced in any material form (including photocopying or storing in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright holder except in accordance with the provisions of the Copyright, Designs and Patents Act 1988. Applications for the copyright holder’s written permission to reproduce any part of this publication should be addressed to the publishers. ISBN 1-897676-13-1 Page layout and design by Nottingham University Press, Nottingham Printed and bound by Bath Press, Bath, England Foreword v Contents Foreword ix T. Pearse Lyons Presient, Alltech Inc., Nicholasville, Kentucky, USA Ethanol industry today 1 Ethanol around the world: rapid growth in policies, technology and production 1 T. Pearse Lyons Alltech Inc., Nicholasville, Kentucky, USA Raw material handling and processing 2 Grain dry milling and cooking procedures: extracting sugars in preparation for fermentation 9 Dave R. Kelsall and T. Pearse Lyons Alltech Inc., Nicholasville, Kentucky, USA 3 Enzymatic conversion of starch to fermentable sugars 23 Ronan F. -

Novel Approach to Introduce Alkyl Chains Into PEDOT:PSS and Its Effect on the Performance As a Flexible Electrode
applied sciences Article Novel Approach to Introduce Alkyl Chains into PEDOT:PSS and Its Effect on the Performance as a Flexible Electrode In-Seong Hwang 1, Chul-Woo Park 1, Hye-In Kang 1, Sung-yoon Joe 2, Na-Young Pak 3 and Dae-won Chung 1,* 1 Department of Polymer Engineering, College of Engineering, The University of Suwon, Hwaseong-si 18323, Korea; [email protected] (I.-S.H.); [email protected] (C.-W.P.); [email protected] (H.-I.K.) 2 Center of Advanced Materials Analysis, The University of Suwon, Hwaseong-si 18323, Korea; [email protected] 3 EverChemTech Co., Ltd., 38, Cheongwonsandan 7-gil, Mado-myeon, Hwaseong-si 18323, Korea; [email protected] * Correspondence: [email protected]; Tel.: +81-312-202-156 Abstract: We here report a synthetic route to introduce alky chains into poly (3,4-ethylenedioxythio- phene):poly (4-styrenesulfonate) (PEDOT:PSS) by the reaction with epoxyalkanes. The reaction was analyzed by FT-IR, TGA, and XPS studies, and the conductivities of derivatives were discussed as a function of the length of alkyl chains. PEDOT:PSS-C6, which is the product from a reaction with epoxyhexane, was well dispersed in methanol and transparent films from this dispersion were successfully prepared. PEDOT:PSS-C6 film showed an increase in hydrophobicity, resulting in enhanced water resistance compared to pristine PEDOT:PSS film, and a morphological study of the film exhibited clear phase separation similar to PEDOT:PSS doped by DMSO. We also observed an improvement in the conductivity and flexibility of PEDOT:PSS-C6 film compared to those of Citation: Hwang, I.-S.; Park, C.-W.; pristine PEDOT:PSS film. -

Amino Mannitol Dehydrogenases on the Azasugar Biosynthetic Pathway
Send Orders for Reprints to [email protected] 10 Protein & Peptide Letters, 2014, 21, 10-14 Medium-Chain Dehydrogenases with New Specificity: Amino Mannitol Dehydrogenases on the Azasugar Biosynthetic Pathway Yanbin Wu, Jeffrey Arciola, and Nicole Horenstein* Department of Chemistry, University of Florida, Gainesville Florida, 32611-7200, USA Abstract: Azasugar biosynthesis involves a key dehydrogenase that oxidizes 2-amino-2-deoxy-D-mannitol to the 6-oxo compound. The genes encoding homologous NAD-dependent dehydrogenases from Bacillus amyloliquefaciens FZB42, B. atrophaeus 1942, and Paenibacillus polymyxa SC2 were codon-optimized and expressed in BL21(DE3) Escherichia coli. Relative to the two Bacillus enzymes, the enzyme from P. polymyxa proved to have superior catalytic properties with a Vmax of 0.095 ± 0.002 mol/min/mg, 59-fold higher than the B. amyloliquefaciens enzyme. The preferred substrate is 2- amino-2-deoxy-D-mannitol, though mannitol is accepted as a poor substrate at 3% of the relative rate. Simple amino alco- hols were also accepted as substrates at lower rates. Sequence alignment suggested D283 was involved in the enzyme’s specificity for aminopolyols. Point mutant D283N lost its amino specificity, accepting mannitol at 45% the rate observed for 2-amino-2-deoxy-D-mannitol. These results provide the first characterization of this class of zinc-dependent medium chain dehydrogenases that utilize aminopolyol substrates. Keywords: Aminopolyol, azasugar, biosynthesis, dehydrogenase, mannojirimycin, nojirimycin. INTRODUCTION are sufficient to convert fructose-6-phosphate into manno- jirimycin [9]. We proposed that the gutB1 gene product was Azasugars such as the nojirimycins [1] are natural prod- responsible for the turnover of 2-amino-2-deoxy-D-mannitol ucts that are analogs of monosaccharides that feature a nitro- (2AM) into mannojirimycin as shown in Fig.