GRAS Notice 789 for Erythritol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

“Polyols: a Primer for Dietetic Professionals” Is a Self-Study
1 “Polyols: A primer for dietetic professionals” is a self-study module produced by the Calorie Control Council, an accredited provider of continuing professional education (CPE) for dietetic professionals by the Commission on Dietetic Registration. It provides one hour of level 1 CPE credit for dietetic professionals. The full text of the module is in the notes section of each page, and is accompanied by summary points and/or visuals in the box at the top of the page. Directions for obtaining CPE are provided at the end of the module. 2 After completing this module, dietetic professionals will be able to: • Define polyols. • Identify the various types of polyols found in foods. • Understand the uses and health effects of polyols in foods. • Counsel clients on how to incorporate polyols into an overall healthful eating pattern. 3 4 Polyols are carbohydrates that are hydrogenated, meaning that a hydroxyl group replaces the aldehyde or ketone group found on sugars. Hydrogenated monosaccharides include erythritol, xylitol, sorbitol, and mannitol. Hydrogenated disaccharides include lactitol, isomalt, and maltitol. And hydrogenated starch hydrolysates (HSH), or polyglycitols (a wide range of corn syrups and maltodextrins), are formed from polysaccharides (Grabitske and Slavin 2008). 5 Nearly 54 percent of Americans are trying to lose weight, more than ever before. Increasingly, they are turning toward no- and low-sugar, and reduced calorie, foods and beverages to help them achieve their weight loss goals (78% of Americans who are trying to lose weight) (CCC 2010). Polyols, found in many of these foods, are becoming a subject of more interest. 6 They are incompletely digested , therefore are sometimes referred to as “low- digestible carbohydrates.” Polyols are not calorie free, as there is some degree of digestion and absorption of the carbohydrate. -
Analysis of Sugars and Sugar Alcohols in Energy Drink by Prominence-I with Differential Refreactive Index Detector
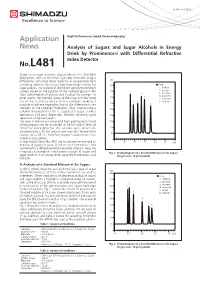
LAAN-A-LC-E258 Application High Performance Liquid Chromatography News Analysis of Sugars and Sugar Alcohols in Energy Drink by Prominence-i with Differential Refractive Index Detector No.L481 Sugars and sugar alcohols display almost no ultraviolet absorption, and are therefore typically detected using a differential refractive index detector or evaporative light uRI scattering detector. By using a ligand exchange column for 80 ■ Peaks sugar analysis, it is possible to distinguish among the different 1. maltose 70 2. glucose isomers based on the position of the hydroxyl group in the 1 4 3. fructose chair conformation of glucose and fructose for example. In 4. erythritol 60 5 other words, the hydroxyl group of the sugar and the metal 5. mannitol ion of the stationary phase form a complex, making it 2 3 6. sorbitol possible to achieve separation due to the difference in the 50 6 strength of the complex formation. Also, maintaining a 40 column temperature of 80 °C suppresses sugar anomer separation and peak dispersion, thereby achieving good 30 separation of adjacent peaks. The new Prominence-i integrated high-performance liquid 20 chromatograph can be connected to the RID-20A differential refractive index detector. The column oven, which can 10 accommodate a 30 cm column and maintain temperature 0 control up to 85 °C, therefore supports applications that require a long column. In Application News No. 467, we introduced an example of 0 5 10 15 20 25 min analysis of sugars in juice, in which the Prominence-i was connected to a differential refractive index detector. Here, we introduce an example of simultaneous analysis of sugars and Fig. -

Low Molecular Weight Organic Composition of Ethanol Stillage from Sugarcane Molasses, Citrus Waste, and Sweet Whey Michael K
Chemical and Biological Engineering Publications Chemical and Biological Engineering 2-1994 Low Molecular Weight Organic Composition of Ethanol Stillage from Sugarcane Molasses, Citrus Waste, and Sweet Whey Michael K. Dowd Iowa State University Steven L. Johansen Iowa State University Laura Cantarella Iowa State University See next page for additional authors Follow this and additional works at: http://lib.dr.iastate.edu/cbe_pubs Part of the Biochemical and Biomolecular Engineering Commons, and the Biological Engineering Commons The ompc lete bibliographic information for this item can be found at http://lib.dr.iastate.edu/ cbe_pubs/12. For information on how to cite this item, please visit http://lib.dr.iastate.edu/ howtocite.html. This Article is brought to you for free and open access by the Chemical and Biological Engineering at Iowa State University Digital Repository. It has been accepted for inclusion in Chemical and Biological Engineering Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Low Molecular Weight Organic Composition of Ethanol Stillage from Sugarcane Molasses, Citrus Waste, and Sweet Whey Abstract Filtered stillage from the distillation of ethanol made by yeast fermentation of sugarcane molasses, citrus waste, and sweet whey was analyzed by gas chromatography/mass spectroscopy and by high-performance liquid chromatography. Nearly all of the major peaks representing low molecular weight organic components were identified. The am jor components in cane stillage were, in decreasing order of concentration, lactic acid, glycerol, ethanol, and acetic acid. In citrus stillage they were lactic acid, glycerol, myo-inositol, acetic acid, chiro-inositol, and proline. -

New Yeasts Capable of Assimilating Methanol*
J. Gen. Appl. Microbiol., 18, 295-305 (1972) NEW YEASTS CAPABLE OF ASSIMILATING METHANOL* TOSHIKAZU OKI, KAGEAKI KOUNO, ATSUO KITAI, ANDASAICHIRO OZAKI Central Research Laboratories of Sanraku- Ocean Co., Ltd., Fujisawa, Japan (Received May 10, 1972) Twenty strains of methanol strongly assimilating yeasts were isolated from rotten tomato and a flower of azalea through investigations on the single- cell protein production and on the microbial utilization of Cl compound. Taxonomic studies indicated that these yeasts were limited to certain species of Candida and Torulopsis, including two new species; C. methanolica OKi et KouNo sp. nov. and T. methanolovescens OKi et KouNo sp. nov. One hundred and ninety-one strains of yeasts obtained from type culture collections did not exhibit methanol assimilability at all. The possibility of producing a single-cell protein from methanol by micro- organisms was suggested by the extensive studies concerning methane- or methanol-assimilating bacteria, Pseudomonas sp. PRL-W4 (1), Pseudomonas methanica (2, 3, 4), Methanomonas methanooxidans (5, 6), Pseudomonas AM 1(7 ), Pseudomonas sp. M27 (8), and Vibrio extorquens (9). Moreover, it is of considerable interest that OGATA et al. first reported the assimila- tion of methanol by yeast, Kloeckera sp. No. 2201 (10, 11, 12). More recently, ASTHANA et al. (13) isolated one species of yeast capable of uti- lizing methanol as a primary carbon source and identified it tentatively as Torulopsis glabrata. However, its taxonomical characteristics have not been reported yet. In the course of investigations on yeast production and microbial utiliza- tion of Ci compounds, two new species of yeasts assimilating methanol as a carbon and energy source were isolated from natural sources. -

United States Patent Office
United States Patentaw Office ... ber of ether groupings on each molecule. These mix tures, however, if they analyze as containing an average 2,978,421. number of ether groups per molecule greater than one, NITRILE COPOLYMERS AND METHOD OF are capable of producing the insoluble nitrile polymers PREPARING SAME of this invention. Since the efficiency of the polyether cross-linking agent increases with the number of poten John A. Holloway, Cleveland, Ohio, assignor to The tially polymerizable groups on the molecule, it is much B. F. Goodrich Company, New York, N.Y., a corpo preferred to utilize polyethers containing an average of ration of New York - two or more alkenyl ether groupings per molecule. The No Drawing. Filed June 9, 1958, Ser. No. 740,566. 10 polyvinyl polyethers of the polyhydric alcohols within the above broad class are produced by reacting acetylene 9 Claims. (C. 260-17.4) with the polyhydric alcohol (or an alcoholate thereof) in a Reppe-type vinylation synthesis. The polycrotyl ethers of the polyhydric alcohols are also useful although they This invention relates to cross-linked alpha-beta ole 15 do not contain a terminal CHCC group. - finically unsaturated nitrile polymers and more particu Illustrative polyhydric alcohols of the above-described larly pertains to copolymers of alpha-beta olefinically class that may be utilized in the preparation of the poly. unsaturated nitriles and polyalkenyl polyethers of poly alkenyl polyether, cross-linking agent include the butane hydric alcohols and methods for their preparation. triols such as 1,2,3-butane triol, 2,3,4-trihydroxybutyric An object of this invention is the provision of insoluble, 20 acid, the aldotetroses such as erythrose and threose, keto cross-linked polynitriles which are capable of thickening tetroses such as erythrulose; the aldopentoses such as certain non-aqueous polar solvents. -

Polyols Have a Variety of Functional Properties That Make Them Useful Alternatives to Sugars in Applications Including Baked Goods
Polyols have a variety of functional properties that make them useful alternatives to sugars in applications including baked goods. Photo © iStockphoto.com/Synergee pg 22 09.12 • www.ift.org BY LYN NABORS and THERESA HEDRICK SUGAR REDUCTION WITH Polyols Polyols are in a unique position to assist with reduced-sugar or sugar-free reformulations since they can reduce calories and complement sugar’s functionality. ugar reduction will be an important goal over the of the product’s original characteristics may still be main- next few years as consumers, government, and in- tained with the replacement of those sugars by polyols. Sdustry alike have expressed interest in lower-calorie In addition, excellent, good-tasting sugar-free products and lower-sugar foods. The 2010 Dietary Guidelines for can be developed by using polyols. Polyols are in a unique Americans put a strong emphasis on consuming fewer position to assist with reduced-sugar or sugar-free refor- calories and reducing intake of added sugars. The In- mulations; since they are only partially digested and ab- stitute of Medicine (IOM) held a public workshop in sorbed, they can reduce calories and complement sugar’s November 2010 to discuss ways the food industry can functionality. Polyols provide the same bulk as sugars and use contemporary and innovative food processing tech- other carbohydrates. Additionally, polyols have a clean, nologies to reduce calorie intake in an effort to reduce sweet taste, which is important since consumers are not and prevent obesity, and in October 2011 recommended likely to sacrifice taste for perceived health benefits. Poly- front-of-package labeling that includes rating the product ols have a host of other functional properties that make based on added sugars content. -

LIQUID-VAPOR EQUILIBRIUM THERMODYNAMICS of the FUSEL OIL: a CASE STUDY Jéssyka Jennifer Miranda Corrêa¹, Edilailsa Januário
LIQUID-VAPOR EQUILIBRIUM THERMODYNAMICS OF THE FUSEL OIL: A CASE STUDY Jéssyka Jennifer Miranda Corrêa¹, Edilailsa Januário de Melo², José Izaquiel Santos da Silva³ ¹Graduating in Chemical Engineering from the Federal University of the Jequitinhonha and Mucuri Valleys, Diamantina - MG, Brazil. ²PhD student in Chemical Engineering, College of Chemical Engineering - State University of Campinas - SP, Brazill. ³Professor at the Federal University of the Jequitinhonha and Mucuri Valleys, Diamantina - MG, Brazil. E-mail for contact: [email protected] Recebido em: 06/04/2019 – Aprovado em: 10/06/2019 – Publicado em: 30/06/2019 DOI: 10.18677/EnciBio_2019A183 ABSTRACT Fusel oil is a by-product of the process of recovering hydrated ethanol, consisting of a mixture of superior alcohols, ethanol, water, among other components. Its commercial interest is mainly due to the presence of isoamyl alcohol, one of the raw materials used in the synthesis of esters, which compounds are relevant for the chemical industry. The recovery of this superior alcohol involves unit operations based on phase equilibrium, which for multicomponent systems can be studied in software that simulates the operation of equipment, in order to present predictions of interactions between its components, reducing errors, time and costs of design. In the industrial, academic and scientific fields, there are numerous challenges regarding the design of separation processes and the monitoring of mixing effects in typical operations, and the Aspen Plus process simulation and optimization platform excels in solving these issues. The current study aims to predict the Liquid-Vapor Equilibrium of the fusel oil, which was considered as a mixture of five components, from the behavioral analysis of each of the binaries that compose it. -

Green Synthesis of 1,5‑Dideoxy‑1,5‑Imino‑Ribitol and 1 ,5
www.nature.com/scientificreports OPEN Green synthesis of 1,5‑dideoxy‑1,5‑imino‑ribitol and 1,5‑ dideox y‑1 ,5‑ imino‑ dl ‑a rabinitol from natural d‑sugars 2− over Au/Al2O3 and SO4 /Al2O3 catalysts Hongjian Gao & Ao Fan* A green synthetic route for the synthesis of some potential enzyme active hydroxypiperidine iminosugars including 1,5‑dideoxy‑1,5‑imino‑ribitol and 1,5‑dideoxy‑1,5‑imino‑dl‑arabinitol, starting from commercially available d‑ribose and d‑lyxose was tested out. Heterogeneous catalysts including 2− Au/Al2O3, SO4 /Al2O3 as well as environmentally friendly reagents were employed into several critical reaction of the route. The synthetic route resulted in good overall yields of 1,5‑dideoxy‑1,5‑imino‑ ribitol of 54%, 1,5‑dideoxy‑1,5‑imino‑d‑arabinitol of 48% and 1,5‑dideoxy‑1,5‑imino‑l‑arabinitol of 46%. The Au/Al2O3 catalyst can be easily recovered from the reaction mixture and reused with no loss of activity. Iminosugars are analogues of carbohydrates, chemically named as polyhydroxylated secondary and tertiary amines and found to be widespread in plants and microorganisms. Tanks to their structural similarity to sugar molecules and excellent metabolic stability, iminosugars are endowed with a high pharmacological potential for a wide range of diseases such as viral infections, tumor metastasis, AIDS, diabetes and lysosomal storage disorders1–11. Iminosugars are generally classifed into fve structural classes: pyrrolidines, piperidines, indolizidines, pyrrolizidines and nortropanes12. Hydroxypiperidines are structurally six-membered iminosugars. Some of the hydroxypiperidines such as 1,5-dideoxy-1,5-iminohexitol derivatives have now been commercialized as drugs to treat type II diabetes mellitus, type I Gaucher disease, Niemann-Pick disease type C (NP-C) and Fabry disease13–18. -

IPOLYHYDRIC ALCOHOLS from WOOD July 1954
IPOLYHYDRIC ALCOHOLS FROM WOOD July 1954 DEC 221954 No. 1984 UNITED STATES DEPARTMENT OF AGRICULTURE --,LFOREST. SERVICE 40REST PRODUCTS LABORATORY÷Q-.---,Y) Madison 5,Wisconsin In Cooperation with the University of Wisconsin 1 POLYHYDRIC ALCOHOLS FROM WOOD-2 2— By J. A. HALL, Director Forest Products Laboratory, 3 Forest Service U. S. Department of Agriculture Wood-sugar solutions produced in the laboratory-scale pilot plant at the U. S. Forest Products Laboratory have been proven to be satis- factory for the production of industrial alcohol, feeding yeast, and wood 4 molasses for stock feed (14, 15, 16, P 17). ± As a continuation of the pro- gram of the Laboratory for developing chemical utilization processes for the wood residues resulting from normal logging operations, and lumber, veneer, and the other forest products manufacture, investiga- tions are in progress to convert wood sugar into merchantable products. Because of the versatility and greatly increased demand for polytlydric alcohols, a study of the production of these materials has been chosen. The alkyd resin industry in the United States has increased tenfold in the past few years and no evidence of decline has been noted. Recently sorbitol has been made available commercially by the catalytic hydro- genation of corn sugar (2). The annual capacity now is 75 million pounds. Sorbitol is widely used as a humectant and conditioner in tobacco, cello- phane, and other products (3). However, one of its fast growing uses is for alkyd resins, where it may replace up to 50 percent glycerine and 25 percent pentaerythritol. This is fortunate since the production of glycerine in the United States as a byproduct of the soap manufacturing 1Presented before the Food and Agriculture Organization of the United Nations, Expert Panel on the Chemistry of Wood, meeting in Stockholm, Sweden, July 27-28, 1953. -

Application of Chiral Sulfoxides in Asymmetric Synthesis
MOJ Bioorganic & Organic Chemistry Review Article Open Access Application of chiral sulfoxides in asymmetric synthesis Abstract Volume 2 Issue 2 - 2018 Chiral sulfoxides are used as a toolbox for the synthesis of enantiomeric/diastereomeric compounds, which are used as precursors for the pharmaceutically/chemically Ganapathy Subramanian Sankaran,1 important molecules. The current review focuses on applying these chiral sulfoxides Srinivasan Arumugan,2 Sivaraman towards the synthesis of the compounds having stereogenic center. In general, the 3 stereogenic center induced by the sulfoxide is able to direct the stereochemistry of Balasubramaniam 1University of Massachusetts Medical School, USA further transformation necessary to complete the total synthesis of bioactive molecules. 2Department of Science and Humanity (Chemistry), Karunya The nature of the reactive conformation of the sulfoxide is strongly dependent on the Institute of Technology and Sciences, India nature of the substituents at C-α and/or C-β. 3Indian Institute of Technology Madras, Chennai, India Correspondence: Sivaraman Balasubramaniam, Senior Research Scientist, Indian Institute of Technology Madras, Chennai, India, Tel +9177 1880 5113, Email [email protected] Received: March 07, 2018 | Published: March 29, 2018 Introduction occurred in a further oxidation step of one of the sulfinyl enantiomer to sulfone.13 The titanium-binaphthol complex catalyzes not only the Over the last three decades, the sulfinyl group has received asymmetric oxidation but also the kinetic -

Sugar Alcohols a Sugar Alcohol Is a Kind of Alcohol Prepared from Sugars
Sweeteners, Good, Bad, or Something even Worse. (Part 8) These are Low calorie sweeteners - not non-calorie sweeteners Sugar Alcohols A sugar alcohol is a kind of alcohol prepared from sugars. These organic compounds are a class of polyols, also called polyhydric alcohol, polyalcohol, or glycitol. They are white, water-soluble solids that occur naturally and are used widely in the food industry as thickeners and sweeteners. In commercial foodstuffs, sugar alcohols are commonly used in place of table sugar (sucrose), often in combination with high intensity artificial sweeteners to counter the low sweetness of the sugar alcohols. Unlike sugars, sugar alcohols do not contribute to the formation of tooth cavities. Common Sugar Alcohols Arabitol, Erythritol, Ethylene glycol, Fucitol, Galactitol, Glycerol, Hydrogenated Starch – Hydrolysate (HSH), Iditol, Inositol, Isomalt, Lactitol, Maltitol, Maltotetraitol, Maltotriitol, Mannitol, Methanol, Polyglycitol, Polydextrose, Ribitol, Sorbitol, Threitol, Volemitol, Xylitol, Of these, xylitol is perhaps the most popular due to its similarity to sucrose in visual appearance and sweetness. Sugar alcohols do not contribute to tooth decay. However, consumption of sugar alcohols does affect blood sugar levels, although less than that of "regular" sugar (sucrose). Sugar alcohols may also cause bloating and diarrhea when consumed in excessive amounts. Erythritol Also labeled as: Sugar alcohol Zerose ZSweet Erythritol is a sugar alcohol (or polyol) that has been approved for use as a food additive in the United States and throughout much of the world. It was discovered in 1848 by British chemist John Stenhouse. It occurs naturally in some fruits and fermented foods. At the industrial level, it is produced from glucose by fermentation with a yeast, Moniliella pollinis. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0165623 A1 Workman Et Al
US 2006O165623A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0165623 A1 Workman et al. (43) Pub. Date: Jul. 27, 2006 (54) NATURAL IDEODORANT COMPOSITION (57) ABSTRACT (75) Inventors: Tanya Workman, Mansonville (CA); Svetlana Ratnikova, Toronto (CA) The present invention relates to a natural deodorant system and a natural system for topical and systemic delivery of Correspondence Address: active ingredients, both systems being primarily free of Louis C. Paul, Esq. preferably substantially free of more preferably essentially CTSW free of, and most preferably completely free of ethoxylates Suite 2400 or other petrochemical derivatives, and comprising: (a) at least one of (1) glycerine (preferably of plant origin), (2) a 420 Lexington Avenue polyol selected from the group consisting of galactitol, New York, NY 10170 (US) erythritol, inositol, ribitol, dithioerythritol, dithiothreitol, (3) a Sugar alcohol, selected from the group consisting of (73) Assignee: Terra Firma Natuals, Inc. mannitol, Sorbitol. Xylitol and maltitol, (4) a hydrogenated starch hydrosylates of at least one of berries, apples or (21) Appl. No.: 11/042,569 plums, and (5) mixtures thereof; (b) water or a lower monohydric alcohol, selected from the group of methanol, (22) Filed: Jan. 24, 2005 ethanol, propanol and isoproponal, or mixtures thereof, present at a combined concentration of at least 20%; (c) one Publication Classification or more carrageenans (preferably of plant origin) or algi nates, or mixtures thereof, present in combined concentra (51) Int. C. tions of less than about 2%; and (d) optionally, one or more A6 IK 8/73 (2006.01) thickeners or gums selected from the group consisting of tara, guar, Xanthan, Arabic, tragacanth, agar, locust bean (52) U.S.