Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde Over Functionalized Multi-Walled Carbon Nanotubes Supported Silver-Cobalt Nanoparticles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amplification of Oxidative Stress by a Dual Stimuli-Responsive Hybrid Drug
ARTICLE Received 11 Jul 2014 | Accepted 12 Mar 2015 | Published 20 Apr 2015 DOI: 10.1038/ncomms7907 Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death Joungyoun Noh1, Byeongsu Kwon2, Eunji Han2, Minhyung Park2, Wonseok Yang2, Wooram Cho2, Wooyoung Yoo2, Gilson Khang1,2 & Dongwon Lee1,2 Cancer cells, compared with normal cells, are under oxidative stress associated with the increased generation of reactive oxygen species (ROS) including H2O2 and are also susceptible to further ROS insults. Cancer cells adapt to oxidative stress by upregulating antioxidant systems such as glutathione to counteract the damaging effects of ROS. Therefore, the elevation of oxidative stress preferentially in cancer cells by depleting glutathione or generating ROS is a logical therapeutic strategy for the development of anticancer drugs. Here we report a dual stimuli-responsive hybrid anticancer drug QCA, which can be activated by H2O2 and acidic pH to release glutathione-scavenging quinone methide and ROS-generating cinnamaldehyde, respectively, in cancer cells. Quinone methide and cinnamaldehyde act in a synergistic manner to amplify oxidative stress, leading to preferential killing of cancer cells in vitro and in vivo. We therefore anticipate that QCA has promising potential as an anticancer therapeutic agent. 1 Department of Polymer Á Nano Science and Technology, Polymer Fusion Research Center, Chonbuk National University, Backje-daero 567, Jeonju 561-756, Korea. 2 Department of BIN Convergence Technology, Chonbuk National University, Backje-daero 567, Jeonju 561-756, Korea. Correspondence and requests for materials should be addressed to D.L. (email: [email protected]). NATURE COMMUNICATIONS | 6:6907 | DOI: 10.1038/ncomms7907 | www.nature.com/naturecommunications 1 & 2015 Macmillan Publishers Limited. -
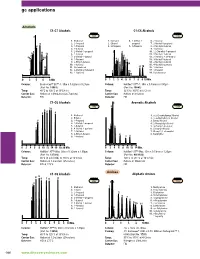
Gc Applications
gc applications Alcohols C1-C7 Alcohols C1-C6 Alcohols 1007 1255 1. Methanol 1. Methanol 4. 2-Methyl-2- 6. 2-Butanol 2. 2-Propanol 2. Ethanol propanol 7. 2-Methyl-1-propanol 3. 1-Propanol 3. 2-Propanol 5. 1-Propanol 8. 2-Methyl-2-butanol 4. 2-Butanol 9. 1-Butanol 5. 2-Methyl-1-propanol 10. 2,2-Dimethyl-1-propanol 6. 1-Butanol 11. 3-Methyl-2-butanol 7. 3-Methyl-1-butanol 12. 2-Pentanol + 3-Pentanol 8. 1-Pentanol 13. 3-Methyl-1-butanol 9. 2-Ethyl-1-butanol 14. 2-Methyl-1-butanol 10. 1-Hexanol 15. 4-Methyl-2-pentanol 11. Cyclohexanol 16. 1-Pentanol 12. 3-Methylcyclohexanol 17. 1-Hexanol 13. 1-Heptanol 18. Cyclohexanol å-IN Column: Econo-Cap™ EC™-1, 30m x 0.32mm x 0.25µm Column: Heliflex® AT™-1, 30m x 0.53mm x 5.00µm (Part No. 19651) (Part No. 16843) Temp: 40°C to 120°C at 10°C/min Temp: 35°C to 100°C at 5°C/min Carrier Gas: Helium at 1.09mL/min (26.7cm/sec) Carrier Gas: Helium at 3mL/min Detector: FID Detector: FID C1-C6 Alcohols Aromatic Alcohols 2596 1249 1. Methanol 1. A,A-Dimethylbenzyl Alcohol 2. Ethanol 2. DL-A-Methylbenzyl Alcohol 3. 1-Propanol 3. Benzyl Alcohol 4. 2-Methyl-1-propanol 4. 2-Phenylethyl Alcohol 5. 1-Butanol 5. 3-Phenyl-1-propanol 6. 4-Methyl-2-pentanol 6. Cinnamyl Alcohol 7. 1-Pentanol 7. Phenyl-1,2-ethanediol 8. 2-Ethyl-1-butanol 8. -

Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like
animals Article Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like Peptide 1 by Interacting with Transient Receptor Potential Ankyrin 1 in a Porcine Ex-Vivo Intestinal Segment Model Elout Van Liefferinge 1,* , Maximiliano Müller 2, Noémie Van Noten 1 , Jeroen Degroote 1 , Shahram Niknafs 2, Eugeni Roura 2 and Joris Michiels 1 1 Laboratory for Animal Nutrition and Animal Product Quality (LANUPRO), Department of Animal Sciences and Aquatic Ecology, Ghent University, 9000 Ghent, Belgium; [email protected] (N.V.N.); [email protected] (J.D.); [email protected] (J.M.) 2 Centre for Nutrition and Food Sciences, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, St. Lucia, QLD 4072, Australia; [email protected] (M.M.); [email protected] (S.N.); [email protected] (E.R.) * Correspondence: [email protected] Simple Summary: The gut is able to “sense” nutrients and release gut hormones to regulate diges- tive processes. Accordingly, various gastrointestinal cell types possess transient receptor potential channels, cation channels involved in somatosensation, thermoregulation and the sensing of pungent and spicy substances. Recent research shows that both channels are expressed in enteroendocrine Citation: Van Liefferinge, E.; Müller, M.; Van Noten, N.; Degroote, J.; cell types responsible for the release of gut peptide hormones such as Cholecystokinin (CCK) and Niknafs, S.; Roura, E.; Michiels, J. Glucagon-like Peptide-1 (GLP-1). A large array of herbal compounds, used in pig nutrition mostly for Cinnamaldehyde Induces Release of their antibacterial and antioxidant properties, are able to activate these channels. Cinnamaldehyde, Cholecystokinin and Glucagon-Like occurring in the bark of cinnamon trees, acts as an agonist of Transient Receptor Potential Ankyrin 1 Peptide 1 by Interacting with (TRPA1)-channel. -

A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing A-Cyano Or A-Ester Functional Group from Baylis-Hillman Adducts
Notes Bull. Korean Chem. Soc. 2004, Vol. 25, No. 3 413 A Practical and Stereoselective Synthesis of Cinnamyl Alcohols Bearing a-Cyano or a-Ester Functional Group from Baylis-Hillman Adducts Ka Young Lee, Saravanan GowriSankar, and Jae Nyoung Kim Department of Chemistry and Institute of Basic Science, Chonnam National University, Gwangju 500-757, Korea Received December 18, 2003 Key Words : Cinnamyl alcohols, Baylis-Hillman adducts, Sulfuric acid, Acetic anhydride Cinnamyl alcohols bearing ester, acid, or nitrile functional Hillman alcohol into the primary acetate or eventually to group at the 2-position are very useful synthons for the primary alcohol (Scheme 1 and Table 1). synthesis of various biologically important compounds.1 The reaction of Baylis-Hillman adducts with acetic Synthesis of cinnamyl alcohol derivatives from the Baylis- anhydride (1.5 equiv.) in the presence of sulfuric acid (10 Hillman adducts has been reported by us and other groups.1-4 mol%) gave the corresponding rearranged cinnamyl acetates The conversion can be carried out directly by using 20% quantitatively in dichloromethane at room temperature aqueous sulfuric acid (for the Baylis-Hillman adducts within 30 min (Actually, the cinnamyl acetate derivatives derived from acrylonitrile)2 or trifluoroacetic acid (for the could be isolated in 92-95% yields.). In order to obtain the Baylis-Hillman adducts derived from acrylates).3 Recently, corresponding cinnamyl alcohols in a one-pot we tried some Basavaiah and coworkers have reported the two-step, one- reaction conditions for the next partial hydrolysis. Addition pot conversion method: TMSOTf-assisted conversion of of water to the reaction mixture (two-phase system) afforded Baylis-Hillman alcohol into the corresponding primary the hydrolyzed cinnamyl alcohols in trace amounts. -

Food and Drug Administration, HHS § 172.515
Food and Drug Administration, HHS § 172.515 Common name Scientific name Limitations Tolu ................................................................ Myroxylon balsamum (L.) Harms. Turpentine ...................................................... Pinus palustris Mill. and other Pinus spp. which yield terpene oils exclusively. Valerian rhizome and roots ............................ Valeriana officinalis L. Veronica ......................................................... Veronica officinalis L .................................................. Do. Vervain, European ......................................... Verbena officinalis L ................................................... Do. Vetiver ............................................................ Vetiveria zizanioides Stapf ......................................... Do. Violet, Swiss ................................................... Viola calcarata L. Walnut husks (hulls), leaves, and green nuts Juglans nigra L. or J. regia L. Woodruff, sweet ............................................. Asperula odorata L ..................................................... In alcoholic beverages only Yarrow ............................................................ Achillea millefolium L .................................................. In beverages only; fin- ished beverage thujone free1 Yerba santa .................................................... Eriodictyon californicum (Hook, et Arn.) Torr. Yucca, Joshua-tree ........................................ Yucca brevifolia Engelm. Yucca, -

Nomination Background: Methyl Trans
' 7/?1 Methyl styryl ketone 122-57-6 NTP NOMINATION HISTORY AND REVIEW A. Nomination History 1. Nomination Source: NCI 2. Recommendations: -Comparative toxicity studies with methyl vinyl ketone -Metabolism -Carcinogenicity 3. Rationale/Remarks: -Potential for widespread human exposure -Nominated as a result of a class study of a,~-unsaturated ketones -Flavoring and fragrance additive -Natural product -Environmental pollutant -Mutagenic in Salmonella 4. Priority: -High for toxicity studies -Moderate for carcinogenicity s. Date of Nomination: July 1994 B. Interagency Committee for Chemical Evaluation and Coordination Review 1. Date of Review: 2. Recommendations: 3. Priority: 4. NTP Chemical Selection Principles: 5. Rationale/Remarks: 122-57-6 Methyl styryl ketone SUMMARY OF DATA FOR CHEMICAL SELECTION CHEMICAL IDENTIFICATION CAS Registry Number: 122-57-6 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl- (SCI, 9CI) Svnonvms and Trade Names: Acetocinnamone; benzalacetone; benzylideneacetone; methyl 2 phenylvinyl ketone; methyl styryl ketone; methyl ~-styryl ketone; 4 phenylbutenone; 4-phenyl-3-butene-2-one; 2-phenylvinyl methyl ketone; styryl methyl ketone; MSK FEMA Number: 2881 Related isomers CAS Registry Number: 937-53-1 Chemica] Abstracts Name: 3-Buten-2-one, 4-phenyl-, (Z)- (SCI, 9CI) Synonvms and Trade Names: cis -4-Phenyl-3-buten-2-one; cis -benzalacetone; cis benzylideneacetone; cis-methyl styryl ketone CAS Registry Number: 1896-62-4 Chemical Abstracts Name: 3-Buten-2-one, 4-phenyl-, (E)- (SCI, 9CI) Synonyms and Trade Names: trans-4-Phenyl-3-buten-2-one; trans-benzalacetone; trans benzylideneacetone; trans-methyl styryl ketone; TPBO Structure. Molecular Formula. and Molecular WeiKbt 0 II H•CHCCH3 Mol. wt.: 146.19 Chemical and Phvsical Properties (from Aldrich Chemical Co., 1993, 1994; Budavari, 1989) Description: White to yellow solid with a sweet, pungent, creamy, floral odor Prepared for NCI by Technical Resources, Inc. -

Trpa1) Activity by Cdk5
MODULATION OF TRANSIENT RECEPTOR POTENTIAL CATION CHANNEL, SUBFAMILY A, MEMBER 1 (TRPA1) ACTIVITY BY CDK5 A dissertation submitted to Kent State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy by Michael A. Sulak December 2011 Dissertation written by Michael A. Sulak B.S., Cleveland State University, 2002 Ph.D., Kent State University, 2011 Approved by _________________, Chair, Doctoral Dissertation Committee Dr. Derek S. Damron _________________, Member, Doctoral Dissertation Committee Dr. Robert V. Dorman _________________, Member, Doctoral Dissertation Committee Dr. Ernest J. Freeman _________________, Member, Doctoral Dissertation Committee Dr. Ian N. Bratz _________________, Graduate Faculty Representative Dr. Bansidhar Datta Accepted by _________________, Director, School of Biomedical Sciences Dr. Robert V. Dorman _________________, Dean, College of Arts and Sciences Dr. John R. D. Stalvey ii TABLE OF CONTENTS LIST OF FIGURES ............................................................................................... iv LIST OF TABLES ............................................................................................... vi DEDICATION ...................................................................................................... vii ACKNOWLEDGEMENTS .................................................................................. viii CHAPTER 1: Introduction .................................................................................. 1 Hypothesis and Project Rationale -

Functional Expression of a Novel Methanol-Stable Esterase From
Cai et al. BMC Biotechnology (2020) 20:36 https://doi.org/10.1186/s12896-020-00622-1 RESEARCH ARTICLE Open Access Functional expression of a novel methanol- stable esterase from Geobacillus subterraneus DSM13552 for biocatalytic synthesis of cinnamyl acetate in a solvent- free system Xianghai Cai1†, Lin Lin2,3†, Yaling Shen1, Wei Wei1* and Dong-zhi Wei1 Abstract Background: Esterases are widely distributed in nature and have important applications in medical, industrial and physiological. Recently, the increased demand for flavor esters has prompted the search of catalysts like lipases and esterases. Esterases from thermophiles also show thermal stability at elevated temperatures and have become enzymes of special interest in biotechnological applications. Although most of esterases catalyzed reactions are carried out in toxic and inflammable organic solvents, the solvent-free system owning many advantages such as low cost and easy downstream processing. Results: The gene estGSU753 from Geobacillus subterraneus DSM13552 was cloned, sequenced and overexpressed into Escherichia coli BL21 (DE3). The novel gene has an open reading frame of 753 bp and encodes 250-amino-acid esterase (EstGSU753). The sequence analysis showed that the protein contains a catalytic triad formed by Ser97, Asp196 and His226, and the Ser of the active site is located in the conserved motif Gly95-X-Ser97-X-Gly99 included in most esterases and lipases. The protein catalyzed the hydrolysis of pNP-esters of different acyl chain lengths, and the enzyme specific activity was 70 U/mg with the optimum substrate pNP-caprylate. The optimum pH and temperature of the recombinant enzyme were 8.0 and 60 °C respectively. -

Chemical Tools for the Synthesis and Analysis of Glycans Thamrongsak Cheewawisuttichai [email protected]
The University of Maine DigitalCommons@UMaine Electronic Theses and Dissertations Fogler Library 8-2019 Chemical Tools for the Synthesis and Analysis of Glycans Thamrongsak Cheewawisuttichai [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/etd Recommended Citation Cheewawisuttichai, Thamrongsak, "Chemical Tools for the Synthesis and Analysis of Glycans" (2019). Electronic Theses and Dissertations. 3058. https://digitalcommons.library.umaine.edu/etd/3058 This Open-Access Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. CHEMICAL TOOLS FOR THE SYNTHESIS AND ANALYSIS OF GLYCANS By Thamrongsak Cheewawisuttichai B.S. Chulalongkorn University, 2012 A DISSERTATION Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy (in Chemistry) The Graduate School The University of Maine August 2019 Advisory Committee: Matthew Brichacek, Assistant Professor of Chemistry, Advisor Alice E. Bruce, Professor of Chemistry Barbara J.W. Cole, Professor of Chemistry Raymond C. Fort, Jr., Professor of Chemistry William M. Gramlich, Associate Professor of Chemistry Copyright 2019 Thamrongsak Cheewawisuttichai ii CHEMICAL TOOLS FOR THE SYNTHESIS AND ANALYSIS OF GLYCANS By Thamrongsak Cheewawisuttichai Dissertation Advisor: Dr. Matthew Brichacek An Abstract of the Dissertation Presented in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy (in Chemistry) August 2019 Glycans can be found in every living organism from plants to bacteria and viruses to human. It has been known that glycans are involved in many biological processes such as structural roles, specific recognition with glycan-binding proteins, and host-pathogen recognitions. -

Proceedings of the Indiana Academy of Science
The Reactions of Silicomolybdic Acid with Organic Compounds 1 John H. Billman, Donald B. Borders, and John A. Buehler, Indiana University, Hondo AFB, Texas, and Anderson College For many years the analytical and inorganic chemists have been seeking specific reagents to identify metallic ions. At the same time, the organic chemist has been interested in finding compounds that would be useful for spotting different types of functional groups commonly en- countered in organic compounds. A typical example of such a reagent is ferric chloride, which has found wide use with phenols. For several years we have been testing various compounds in an effort to find reagents that would be specific for metallic ions as well as organic functional groups. In the course of our investigation it was found that silicomolybdic acid (1), H 4 SiMo 12 O 40 .xH 2 O, would produce a dark blue mixture in the presence of aliphatic aldehydes, but would not give the same test with aromatic aldehydes. Further investigation indi- cated that only aldehydes that contain a hydrogen atom on the alpha carbon atom would give a positive test. On the basis of this work a more thorough study was undertaken of the reaction of silicomolybdic acid with various organic compounds containing different functional groups. Altogether the reagent has been tried with a one-hundred and forty-five organic compounds. Table 1 shows the aldehydes that have been tested. TABLE I Aldehydes — Formaldehyde — Benzaldehyde + Acetaldehyde — m-Nitrobenzaldehyde -f Phenylacetaldehyde — p-Nitrobenzaldehyde -

United States Patent Office Patented Dec
3,293,045 United States Patent Office Patented Dec. 20, 1966 2 vide wintergreen-flavored candies which contain as their 3,293,045 main flavoring ingredient wintergreen oil in lower mini INCREASING THE FLAVOR STRENGTH OF ANE THOLE, CENNAMALDEHYDE AND METHY mum effective levels than have previously been employed. SALICYLATE WITH MALTOL These and other objects are readily achieved through Joan M. Griffin, Forest Hills, N.Y., assignor to Chas. use of the compositions of the instant invention which are, Pfizer & Co., Inc., New York, N.Y., a corporation of in essence: A flavoring composition comprising an agent Delaware Selected from the group consisting of anethole, cinna No Drawing. Filed Oct. 18, 1963, Ser. No. 317,126 maldehyde and methyl salicylate and from about 15% 3 Claims. (C. 99-134) to about 100% by weight thereof of maltol. The instant invention contemplates the use of both This application relates to new and novel flavoring O natural and Synthetic anise, cinnamon and wintergreen compositions. More particularly, it concerns composi oils. It contemplates their use in the form of pure oils tions comprising aromatic flavoring ingredients together or blends of pure oils prepared either synthetically, being with maltol, a gamma-pyrone. There are also contem obtained by chemical reaction and distillation or, natural plated foods, beverages, candy and tablets, syrups, medi 5 ly, being obtained by extraction from the plant material cinal oils, pill and tablet coatings and troches containing from which the Subject oils have been classically isolated. these flavoring compositions. Generally speaking, the natural oils will, as described Among the most useful items of commerce are natural hereinafter, comprise predominantly one chemical entity and Synthetic flavors of the type represented by anise, together with isomers of this entity and minor amounts cinnamon and wintergreen. -

Method TO-11A
EPA/625/R-96/010b Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-11A Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC) [Active Sampling Methodology] Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 January 1999 Method TO-11A Acknowledgements This Method was prepared for publication in the Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition (EPA/625/R-96/010b), which was prepared under Contract No. 68-C3-0315, WA No. 3-10, by Midwest Research Institute (MRI), as a subcontractor to Eastern Research Group, Inc. (ERG), and under the sponsorship of the U.S. Environmental Protection Agency (EPA). Justice A. Manning, John O. Burckle, and Scott Hedges, Center for Environmental Research Information (CERI), and Frank F. McElroy, National Exposure Research Laboratory (NERL), all in the EPA Office of Research and Development, were responsible for overseeing the preparation of this method. Additional support was provided by other members of the Compendia Workgroup, which include: • John O. Burckle, U.S. EPA, ORD, Cincinnati, OH • James L. Cheney, Corps of Engineers, Omaha, NB • Michael Davis, U.S. EPA, Region 7, KC, KS • Joseph B. Elkins Jr., U.S. EPA, OAQPS, RTP, NC • Robert G. Lewis, U.S. EPA, NERL, RTP, NC • Justice A. Manning, U.S. EPA, ORD, Cincinnati, OH • William A. McClenny, U.S. EPA, NERL, RTP, NC • Frank F. McElroy, U.S. EPA, NERL, RTP, NC • Heidi Schultz, ERG, Lexington, MA • William T.