Chem. Pharm. Bull. 53(5) 457—470 (2005) 457 Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrophysiologic Evidence for Increased Endogenous Gabaergic but Not Glycinergic Inhibitory Tone in the Rat Spinal Nerve Ligation Model of Neuropathy Vesa K
Anesthesiology 2001; 94:333–9 © 2001 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Electrophysiologic Evidence for Increased Endogenous GABAergic but Not Glycinergic Inhibitory Tone in the Rat Spinal Nerve Ligation Model of Neuropathy Vesa K. Kontinen, M.D., Ph.D.,* Louise C. Stanfa, Ph.D.,† Amlan Basu,‡ Anthony H. Dickenson, Ph.D.§ Background: Changes in the inhibitory activity mediated by There is evidence that both GABA and glycinergic inter- ␥-aminobutyric acid (GABA) and glycine, acting at spinal GABA A neurons are preferentially associated with the control of receptors and strychnine-sensitive glycine receptors, are of in- 6–9 terest in the development of neuropathic pain. There is ana- low-threshold afferent input to the spinal cord. Con- Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/94/2/333/402482/0000542-200102000-00024.pdf by guest on 29 September 2021 tomic evidence for changes in these transmitter systems after sistent with this, intrathecal administration of the 10 nerve injuries, and blocking either GABAA or glycine receptors GABAA-receptor antagonist bicuculline or the glycine- has been shown to produce allodynia-like behavior in awake receptor antagonist strychnine10,11 produces segmen- normal animals. tally localized tactile allodynia-like behavior in conscious Methods: In this study, the possible changes in GABAergic and glycinergic inhibitory activity in the spinal nerve ligation rats and increased responses to mechanical stimulation 12 model of neuropathic pain were studied by comparing the in anesthetized cats. Intrathecal strychnine has also effects of the GABAA-receptor antagonist bicuculline and the been shown to produce in anesthetized rats reflex re- glycine-receptor antagonist strychnine in neuropathic rats to sponses similar to those produced by nociceptive stim- their effects in sham-operated and nonoperated control rats. -

Stilbene Synthesis by Mizoroki–Heck Coupling Reaction Under Microwave Irradiation
An effective Pd nanocatalyst in aqueous media: stilbene synthesis by Mizoroki–Heck coupling reaction under microwave irradiation Carolina S. García, Paula M. Uberman and Sandra E. Martín* Full Research Paper Open Access Address: Beilstein J. Org. Chem. 2017, 13, 1717–1727. INFIQC-CONICET- Universidad Nacional de Córdoba, Departamento doi:10.3762/bjoc.13.166 de Química Orgánica, Facultad de Ciencias Químicas, Haya de la Torre y Medina Allende, Ciudad Universitaria, X5000HUA, Córdoba, Received: 25 April 2017 Argentina Accepted: 04 August 2017 Published: 18 August 2017 Email: Sandra E. Martín* - [email protected] Associate Editor: L. Vaccaro * Corresponding author © 2017 García et al.; licensee Beilstein-Institut. License and terms: see end of document. Keywords: aqueous reaction medium; MAOS; Mizoroki–Heck reaction; Pd nanoparticle; sustainable organic synthesis Abstract Aqueous Mizoroki–Heck coupling reactions under microwave irradiation (MW) were carried out with a colloidal Pd nanocatalyst stabilized with poly(N-vinylpyrrolidone) (PVP). Many stilbenes and novel heterostilbenes were achieved in good to excellent yields starting from aryl bromides and different olefins. The reaction was carried out in a short reaction time and with low catalyst loading, leading to high turnover frequency (TOFs of the order of 100 h−1). The advantages like operational simplicity, high robust- ness, efficiency and turnover frequency, the utilization of aqueous media and simple product work-up make this protocol a great option for stilbene syntheses by Mizoroki–Heck reaction. Introduction Palladium-catalyzed reactions have emerged as an important basic types of Pd-catalyzed reactions, particularly the vinyl- tool for organic synthesis. Among them, cross-coupling and ation of aryl/vinyl halides or triflates [7-10], explored not only coupling reactions have been broadly applied for C–C and in the inter- and intramolecular version [2,11-13], but also in C–heteroatom bond formation [1-6]. -
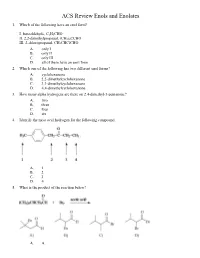
Ochem ACS Review 18 Enols and Enolates
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Unnamed Document
Mutations M287L and Q266I in the Glycine Receptor ␣1 Subunit Change Sensitivity to Volatile Anesthetics in Oocytes and Neurons, but Not the Minimal Alveolar Concentration in Knockin Mice Cecilia M. Borghese, Ph.D.,* Wei Xiong, Ph.D.,† S. Irene Oh, B.S.,‡ Angel Ho, B.S.,§ S. John Mihic, Ph.D.,ʈ Li Zhang, M.D.,# David M. Lovinger, Ph.D.,** Gregg E. Homanics, Ph.D.,†† Edmond I. Eger 2nd, M.D.,‡‡ R. Adron Harris, Ph.D.§§ ABSTRACT What We Already Know about This Topic • Inhibitory spinal glycine receptor function is enhanced by vol- Background: Volatile anesthetics (VAs) alter the function of atile anesthetics, making this a leading candidate for their key central nervous system proteins but it is not clear which, immobilizing effect if any, of these targets mediates the immobility produced by • Point mutations in the ␣1 subunit of glycine receptors have been identified that increase or decrease receptor potentiation VAs in the face of noxious stimulation. A leading candidate is by volatile anesthetics the glycine receptor, a ligand-gated ion channel important for spinal physiology. VAs variously enhance such function, and blockade of spinal glycine receptors with strychnine af- fects the minimal alveolar concentration (an anesthetic What This Article Tells Us That Is New EC50) in proportion to the degree of enhancement. • Mice harboring specific mutations in their glycine receptors Methods: We produced single amino acid mutations into that increased or decreased potentiation by volatile anesthetic in vitro did not have significantly altered changes in anesthetic the glycine receptor ␣1 subunit that increased (M287L, third potency in vivo transmembrane region) or decreased (Q266I, second trans- • These findings indicate that this glycine receptor does not me- membrane region) sensitivity to isoflurane in recombinant diate anesthetic immobility, and that other targets must be receptors, and introduced such receptors into mice. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -

Environmental Properties of Chemicals Volume 2
1 t ENVIRONMENTAL 1 PROTECTION Esa Nikunen . Riitta Leinonen Birgit Kemiläinen • Arto Kultamaa Environmental properties of chemicals Volume 2 1 O O O O O O O O OO O OOOOOO Ol OIOOO FINNISH ENVIRONMENT INSTITUTE • EDITA Esa Nikunen e Riitta Leinonen Birgit Kemiläinen • Arto Kultamaa Environmental properties of chemicals Volume 2 HELSINKI 1000 OlO 00000001 00000000000000000 Th/s is a second revfsed version of Environmental Properties of Chemica/s, published by VAPK-Pub/ishing and Ministry of Environment, Environmental Protection Department as Research Report 91, 1990. The pubiication is also available as a CD ROM version: EnviChem 2.0, a PC database runniny under Windows operating systems. ISBN 951-7-2967-2 (publisher) ISBN 952-7 1-0670-0 (co-publisher) ISSN 1238-8602 Layout: Pikseri Julkaisupalvelut Cover illustration: Jussi Hirvi Edita Ltd. Helsinki 2000 Environmental properties of chemicals Volume 2 _____ _____________________________________________________ Contents . VOLUME ONE 1 Contents of the report 2 Environmental properties of chemicals 3 Abbreviations and explanations 7 3.1 Ways of exposure 7 3.2 Exposed species 7 3.3 Fffects________________________________ 7 3.4 Length of exposure 7 3.5 Odour thresholds 8 3.6 Toxicity endpoints 9 3.7 Other abbreviations 9 4 Listofexposedspecies 10 4.1 Mammais 10 4.2 Plants 13 4.3 Birds 14 4.4 Insects 17 4.5 Fishes 1$ 4.6 Mollusca 22 4.7 Crustaceans 23 4.8 Algae 24 4.9 Others 25 5 References 27 Index 1 List of chemicals in alphabetical order - 169 Index II List of chemicals in CAS-number order -

Traditional Medicine
MINISTRY OF HEALTH DEPARTMENT OF MEDICAL RESEARCH (LOWER MYANMAR) -4 rf,"d .1, l,.ifr M '\t $.,iJ+j AI{I{OTATED BIBLIOGRAPHY OF TRADITIOI{AL MEDTCII\E RESEARCH CARRTED OUT AT DMR (LM) nURIf{G 196s-2011 f# #a# €€# 6rdffi t u 6 l6'6 ktibilicetidiT \ &, ft Ministry of Health Department of Medical Research (Lower Myanmar) Central Biomedical Library ANNOTATED BIBLIOGRAPHY OF TRADITIONAL MEDICINE RESEARCH CARRIED OUT AT DMR (LM) DURING 1965-2011 Compiled by Cho Mar Oo BA (Economics); DipLibSc Librarian, Central Biomedical Library Staff of Central Biomedical Library May Aye Than MBBS, MMedSc (Pharmacology) Deputy Director & Head Pharmacology Research Division Staff of Pharmacology Research Division Aung Myo Min BSc (Physics); DipLibSc; RL Librarian & Head (Retd.) Central Biomedical Library Ye Htut MBBS, MSc (Medical Parasitology) (London) DLSHTM, FRCP (Edin) Deputy Director-General Myo Khin MBBS, MD (New South Wales), DCH, FRCP (Edin) Acting Director General PREFACE Throughout recorded history, people of various cultures have relied on traditional medicine. Worldwide, only an estimated ten to thirty percent of human health care is delivered by conventional, biomedically oriented practitioners. The remaining seventy to ninety percent ranges from self-care according to folk principles, to care given in an organized health care system based on traditional medicine. Likewise, in Myanmar health care system, the existence of traditional medicine along with allopathic medicines is well recognized. Myanmar traditional medicine dates back 2,000 years and is well accepted and widely used by the people throughout history. Burma Medical Research Institute since it was established in 1963 had started a program of research on traditional medicinal plants including laboratory screening tests on animal models of herbs with reputed pharmacological properties-such as anti-dysentery, bronchodilator, hypoglycemic effects. -

Oxidation of Secondary Alcohols to Ketones
Oxidation of secondary alcohols to ketones The oxidation of secondary alcohols to ketones is an important oxidation reaction in organic chemistry. Where a secondary alcohol is oxidised, it is converted to a ketone. The hydrogen from the hydroxyl group is lost along with the hydrogen bonded to the second carbon. The remaining oxygen then forms double bonds with the carbon. This leaves a ketone, as R1–COR2. Ketones cannot normally be oxidised any further because this would involve breaking a C–C bond, which requires too much energy.[1] The reaction can occur using a variety of oxidants. Contents Potassium dichromate PCC (Pyridinium chlorochromate) Dess–Martin oxidation Swern oxidation Oppenauer oxidation Fétizon oxidation See also References Potassium dichromate A secondary alcohol can be oxidised into a ketone using acidified potassium dichromate and heating under 2− 3+ reflux. The orange-red dichromate ion, Cr2O7 , is reduced to the green Cr ion. This reaction was once used in an alcohol breath test. PCC (Pyridinium chlorochromate) PCC, when used in an organic solvent, can be used to oxidise a secondary alcohol into a ketone. It has the advantage of doing so selectively without the tendency to over-oxidise. Dess–Martin oxidation The Dess–Martin periodinane is a mild oxidant for the conversion of alcohols to aldehydes or ketones.[2] The reaction is performed under standard conditions, at room temperature, most often in dichloromethane. The reaction takes between half an hour and two hours to complete. The product is then separated from the spent periodinane.[3] Swern oxidation Swern oxidation oxidises secondary alcohols into ketones using oxalyl chloride and dimethylsulfoxide. -

Mechanisms of the Mizoroki-Heck Reaction
P1: OTA c01 JWBK261-Oestreich December 16, 2008 10:9 Printer: Yet to come 1 Mechanisms of the Mizoroki–Heck Reaction Anny Jutand Departement´ de Chimie, Ecole Normale Superieure,´ CNRS, 24 Rue Lhomond, Paris Cedex 5, France 1.1 Introduction The palladium-catalysed Mizoroki–Heck reaction is the most efficient route for the vinyla- tion of aryl/vinyl halides or triflates. This reaction, in which a C C bond is formed, proceeds in the presence of a base (Scheme 1.1) [1, 2]. Nonconjugated alkenes are formed in re- actions involving cyclic alkenes (Scheme 1.2) [1e, 2a,c,e,g] or in intramolecular reactions (Scheme 1.3) [2b,d–g] with creation of stereogenic centres. Asymmetric Mizoroki–Heck reactions may be performed in the presence of a chiral ligand [2]. The Mizoroki–Heck reaction has been intensively developed from a synthetic and mechanistic point of view, as expressed by the impressive number of reviews and book chapters [1, 2]. In the late 1960s, Heck reported that arylated alkenes were formed in the reaction of alkenes with a stoichiometric amount of [Ar–Pd Cl] or [Ar–Pd–OAc], generated in situ by reacting ArHgCl with PdCl2 or ArHgOAc with Pd(OAc)2 respectively [3]. A mechanism was proposed which involves a syn migratory insertion of the alkene into the Ar–Pd bond, followedbyasyn β-hydride elimination of a hydridopalladium [HPdX] (X = Cl, OAc) (Scheme 1.4a). In the case of cyclic alkenes, in which no syn β-hydride is available, a syn β-hydride elimination occurs, leading to a nonconjugated alkene (Scheme 1.4b). -

Alcohol Oxidation
Alcohol oxidation Alcohol oxidation is an important organic reaction. Primary alcohols (R-CH2-OH) can be oxidized either Mechanism of oxidation of primary alcohols to carboxylic acids via aldehydes and The indirect oxidation of aldehyde hydrates primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (R- CH(OH)2) by reaction with water. The oxidation of a primary alcohol at the aldehyde level is possible by performing the reaction in absence of water, so that no aldehyde hydrate can be formed. Contents Oxidation to aldehydes Oxidation to ketones Oxidation to carboxylic acids Diol oxidation References Oxidation to aldehydes Oxidation of alcohols to aldehydes is partial oxidation; aldehydes are further oxidized to carboxylic acids. Conditions required for making aldehydes are heat and distillation. In aldehyde formation, the temperature of the reaction should be kept above the boiling point of the aldehyde and below the boiling point of the alcohol. Reagents useful for the transformation of primary alcohols to aldehydes are normally also suitable for the oxidation of secondary alcohols to ketones. These include: Oxidation of alcohols to aldehydes and ketones Chromium-based reagents, such as Collins reagent (CrO3·Py2), PDC or PCC. Sulfonium species known as "activated DMSO" which can result from reaction of DMSO with electrophiles, such as oxalyl chloride (Swern oxidation), a carbodiimide (Pfitzner-Moffatt oxidation) or the complex SO3·Py (Parikh-Doering oxidation). Hypervalent iodine compounds, such as Dess-Martin periodinane or 2-Iodoxybenzoic acid. Catalytic TPAP in presence of excess of NMO (Ley oxidation). Catalytic TEMPO in presence of excess bleach (NaOCl) (Oxoammonium-catalyzed oxidation). -

Regiocontrol in the Heck-Reaction and Fast Fluorous Chemistry
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 244 _____________________________ _____________________________ Regiocontrol in the Heck-Reaction and Fast Fluorous Chemistry BY KRISTOFER OLOFSSON ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2001 Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Organic Pharmaceutical Chemistry presented at Uppsala University in 2001. Abstract Olofsson, K. 2001. Regiocontrol in the Heck-Reaction and Fast Fluorous Chemistry. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 244. 74 pp. Uppsala. ISBN 91–554–4883–6 The palladium-catalysed Heck-reaction has been utilised in organic synthesis, where the introduction of aryl groups at the internal, β-carbon of different allylic substrates has been performed with high regioselectivity. The β-stabilising effect of silicon enhances the regiocontrol in the internal arylation of allyltrimethylsilane, while a coordination between palladium and nitrogen induces very high regioselectivities in the arylation of N,N-dialkylallylamines and the Boc-protected allylamine, producing β-arylated arylethylamines, which are of interest for applications in medicinal chemistry. Phthalimido-protected allylamines are arylated with poor to moderate regioselectivity. Single-mode microwave heating can reduce the reaction times of Heck-, Stille- and radical mediated reactions drastically from approximately 20 hours to a few minutes with, in the majority of cases, retained, high -

Benzyl Ligand† Cite This: Chem
ChemComm View Article Online COMMUNICATION View Journal | View Issue Homoleptic organolanthanide compounds supported by the bis(dimethylsilyl)benzyl ligand† Cite this: Chem. Commun., 2017, 53,716 Kasuni C. Boteju, Arkady Ellern and Aaron D. Sadow* Received 21st November 2016, Accepted 12th December 2016 DOI: 10.1039/c6cc09304c www.rsc.org/chemcomm À A b-SiH functionalized benzyl anion [C(SiHMe2)2Ph] is obtained by A strategy for stabilizing coordinatively unsaturated rare earth deprotonation of HC(SiHMe2)2Ph with KCH2Ph or by reaction of amides has involved the incorporation of SiH groups, which 14 KOtBu and (Me2HSi)3CPh; LnI3(THF)n and three equivalents of this form labile secondary interactions with the lanthanide center. carbanion combine to provide homoleptic tris(alkyl)lanthanide Furthermore, the SiH moiety provides a powerful signature in 1 29 Creative Commons Attribution 3.0 Unported Licence. compounds Ln{C(SiHMe2)2Ph}3 (Ln = La, Ce, Pr, Nd) containing Hand Si NMR and IR spectra. This b-SiH strategy may also be secondary metal–ligand interactions. applied to alkyls, and the ligand C(SiHMe2)3 supports trivalent yttrium and divalent ytterbium and samarium homoleptic alkyls Synthesis of homoleptic organolanthanide complexes, particu- containing secondary Ln(H–Si interactions.15,16 Recently, we larly those of the early trivalent lanthanides (La–Nd), is challenging reported Ce{C(SiHMe2)3}3 as a precursor to a zwitterionic hydro- due to the large radii of these elements, polar bonding, high silylation catalyst.17 New chemistry might be accessed with alkyl charge, and high Lewis acidity.1 Such homoleptic compounds ligand variations that include both b-SiH and benzylic function- should be valuable for the synthesis of new catalysts and alities, and these groups could compete to enhance the homo- new materials,2 yet solvent- or donor-group-free, salt-free, and leptic compounds’ resistance to undesired ligand elimination This article is licensed under a thermally robust organolanthanide compounds are not readily pathways.