Cooley, J. R. 1999. Sexual Behavior in North American Cicadas of The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First New Zealand Insects Collected on Cook's
Pacific Science (1989), vol.43, 43, nono.. 1 © 1989 by UniversityUniversity of Hawaii Press.Pres s. All rights reserved TheThe First New Zealand Zealand InsectsInsects CollectedCollectedon Cook'sCook's Endeavour Voyage!Voyage! 2 J. R. H. AANDREWSNDREWS2 AND G.G . W. GIBBSGmBS ABSTRACT:ABSTRACT: The Banks collection of 40 insect species, species, described by J. J. C.C. Fabricius in 1775,1775, is critically examined to explore the possible methods of collection and to document changesto the inseinsectct fauna andto the original collection localities sincsincee 1769.The1769. The aassemblagessemblageof species is is regarded as unusual. unusual. It includes insects that are large large and colorful as well as those that are small and cryptic;cryptic; some species that were probably common were overlooked, but others that are today rare were taken.taken. It is concluded that the Cook naturalists caught about 15species with a butterfly net, but that the majority (all CoColeoptera)leoptera) were discoveredin conjunction with other biobiologicallogical specimens, especially plantsplants.. PossibPossiblele reasons for the omission ofwetwetasas,, stick insects, insects, etc.,etc., are discussed. discussed. This early collection shows that marked changesin abundance may have occurred in some speciespeciess since European colonizationcolonization.. One newrecord is is revealed:revealed: The cicada NotopsaltaNotopsaltasericea sericea (Walker) was found to be among the Fabricius specispeci mens from New Zealand,Zealand, but itsits description evidentlyevidently -
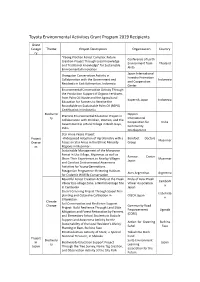
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

Supporting Information
Supporting Information Campbell et al. 10.1073/pnas.1421386112 SI Materials and Methods transformed into Escherichia coli JM109 High Efficiency Com- Genome Sequencing. The following amount of data were generated petent Cells. Transformed cells were grown in 3 mL of LB broth at for each sequencing technology: 136,081,956 pairs of 100 × 2 37 °C overnight, and plasmids were purified with E.Z.N.A. plas- short insert Illumina HiSeq reads for about 27 Gb total; 50,884,070 mid DNA mini kit I. The purified plasmids were used as PCR pairs of 100 × 2 large insert HiSeq reads for about 10 Gb total; templates to for further amplification of the probe region. The and 259,593 reads averaging 1600 nt for about 421 Mb total of amplified probes were subject to nick translation (>175 ng/μL PacBio data. DNA, 1× nick-translation buffer, 0.25 mM unlabeled dNTPs, 50 μM labeled dNTPs, 2.3 U/μL DNA polymerase I, 9 mU/μL Genome Annotation. Annotation of Hodgkinia DNA was done Dnase), using either Cy3 (MAGTRE006 and MAGTRE005), or using the phmmer module of HMMER v3.1b1 (1). All ORFs Cy5 (MAGTRE001 and MAGTRE012), and size selected for beginning with a start codon that overlapped a phmmer hit sizes in the range of 100–500 bp using Ampure XP beads. Probes were searched against a database of all Hodgkinia genes using with at least seven incorporated labeled dNTPs per 1,000 nucle- BLASTX 2.2.28+. MAGTRE Hodgkinia genes were considered otides as determined by spectroscopy were used for hybridization. -

Diversity of Entomopathogens Fungi: Which Groups Conquered
bioRxiv preprint doi: https://doi.org/10.1101/003756; this version posted April 4, 2014. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Diversity of entomopathogens Fungi: Which groups conquered the insect body? João P. M. Araújoa & David P. Hughesb aDepartment of Biology, Penn State University, University Park, Pennsylvania, United States of America. bDepartment of Entomology and Department of Biology, Penn State University, University Park, Pennsylvania, United States of America. [email protected]; [email protected]; Abstract The entomopathogenic Fungi comprise a wide range of ecologically diverse species. This group of parasites can be found distributed among all fungal phyla and as well as among the ecologically similar but phylogenetically distinct Oomycetes or water molds, that belong to a different kingdom (Stramenopila). As a group, the entomopathogenic fungi and water molds parasitize a wide range of insect hosts from aquatic larvae in streams to adult insects of high canopy tropical forests. Their hosts are spread among 18 orders of insects, in all developmental stages such as: eggs, larvae, pupae, nymphs and adults exhibiting completely different ecologies. Such assortment of niches has resulted in these parasites evolving a considerable morphological diversity, resulting in enormous biodiversity, much of which remains unknown. Here we gather together a huge amount of records of these entomopathogens to comparing and describe both their morphologies and ecological traits. These findings highlight a wide range of adaptations that evolved following the evolutionary transition to infecting the most diverse and widespread animals on Earth, the insects. -

Evidence for Paternal Leakage in Hybrid Periodical Cicadas (Hemiptera: Magicicada Spp.) Kathryn M
Evidence for Paternal Leakage in Hybrid Periodical Cicadas (Hemiptera: Magicicada spp.) Kathryn M. Fontaine¤, John R. Cooley*, Chris Simon Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, Connecticut, United States of America Mitochondrial inheritance is generally assumed to be maternal. However, there is increasing evidence of exceptions to this rule, especially in hybrid crosses. In these cases, mitochondria are also inherited paternally, so ‘‘paternal leakage’’ of mitochondria occurs. It is important to understand these exceptions better, since they potentially complicate or invalidate studies that make use of mitochondrial markers. We surveyed F1 offspring of experimental hybrid crosses of the 17-year periodical cicadas Magicicada septendecim, M. septendecula, and M. cassini for the presence of paternal mitochondrial markers at various times during development (1-day eggs; 3-, 6-, 9-week eggs; 16-month old 1st and 2nd instar nymphs). We found evidence of paternal leakage in both reciprocal hybrid crosses in all of these samples. The relative difficulty of detecting paternal mtDNA in the youngest eggs and ease of detecting leakage in older eggs and in nymphs suggests that paternal mitochondria proliferate as the eggs develop. Our data support recent theoretical predictions that paternal leakage may be more common than previously estimated. Citation: Fontaine KM, Cooley JR, Simon C (2007) Evidence for Paternal Leakage in Hybrid Periodical Cicadas (Hemiptera: Magicicada spp.). PLoS ONE 2(9): e892. doi:10.1371/journal.pone.0000892 INTRODUCTION The seven currently-recognized 13- and 17-year periodical Although mitochondrial DNA (mtDNA) exhibits a variety of cicada species (Magicicada septendecim {17}, M. tredecim {13}, M. -

And Mushroom-Associated Alkaloids from Two Behavior Modifying
bioRxiv preprint doi: https://doi.org/10.1101/375105; this version posted December 18, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying 2 cicada pathogens 3 4 Greg R. Boyce1, Emile Gluck-Thaler2, Jason C. Slot2, Jason E. Stajich3, William J. Davis4, Tim 5 Y. James4, John R. Cooley5, Daniel G. Panaccione1, Jørgen Eilenberg6, Henrik H. De Fine Licht6, 6 Angie M. Macias1, Matthew C. Berger1, Kristen L. Wickert1, Cameron M. Stauder1, Ellie J. 7 Spahr1, Matthew D. Maust1, Amy M. Metheny1, Chris Simon5, Gene Kritsky7, Kathie T. Hodge8, 8 Richard A. Humber8,9, Terry Gullion10, Dylan P. G. Short11, Teiya Kijimoto1, Dan Mozgai12, 9 Nidia Arguedas13, Matt T. Kasson1,* 10 11 1Division of Plant and Soil Sciences, West Virginia University, Morgantown, West Virginia, 12 26506, USA. 13 2Department of Plant Pathology, The Ohio State University, Columbus, Ohio 43210, USA. 14 3Department of Microbiology and Plant Pathology and Institute for Integrative Genome Biology, 15 University of California, Riverside, California 92521, USA. 16 4Department of Ecology and Evolution, University of Michigan, Ann Arbor, Michigan 48109, 17 USA. 18 5Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, 19 Connecticut 06269, USA. 20 6Department of Plant and Environmental Science, University of Copenhagen, Denmark. 21 7Department of Biology, Mount St. Joseph University, Cincinnati, Ohio 45233, USA. 22 8Plant Pathology & Plant-Microbe Biology, School of Integrative Plant Science, Cornell 23 University, Ithaca, New York 14853, USA. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

The Evolutionary Biology of Herbivorous Insects
GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 1 Aptara (PPG-Quark) PART I EVOLUTION OF POPULATIONS AND SPECIES GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 2 Aptara (PPG-Quark) GRBQ316-3309G-C01[01-19].qxd 7/17/07 12:07 AM Page 3 Aptara (PPG-Quark) ONE Chemical Mediation of Host-Plant Specialization: The Papilionid Paradigm MAY R. BERENBAUM AND PAUL P. FEENY Understanding the physiological and behavioral mecha- chemistry throughout the life cycle are central to these nisms underlying host-plant specialization in holo- debates. Almost 60 years ago, Dethier (1948) suggested that metabolous species, which undergo complete development “the first barrier to be overcome in the insect-plant relation- with a pupal stage, presents a particular challenge in that ship is a behavioral one. The insect must sense and discrim- the process of host-plant selection is generally carried out inate before nutritional and toxic factors become opera- by the adult stage, whereas host-plant utilization is more tive.” Thus, Dethier argued for the primacy of adult [AQ2] the province of the larval stage (Thompson 1988a, 1988b). preference, or detection and response to kairomonal cues, Thus, within a species, critical chemical, physical, or visual in host-plant shifts. In contrast, Ehrlich and Raven (1964) cues for host-plant identification may differ over the course reasoned that “after the restriction of certain groups of of the life cycle. An organizing principle for the study of insects to a narrow range of food plants, the formerly repel- host-range evolution is the preference-performance hypoth- lent substances of these plants might . -

Forest and Timber Insects in New Zealand No
Forest and Timber Insects in New Zealand No. 44 Large Cicadas Insect: Amphipsalta zelandica (Boisduval) (Hemiptera: Cicadidae) Amphipsalta cingulata (Fabricius) Amphipsalta strepitans (Kirkaldy) Based on M. K. Kay (1980) Fig. 1 - Adult Amphipsalta zelandica male. Type of injury Young cicadas (nymphs) and adults both have piercing-sucking mouth- parts with which they take up plant sap. Nymphs live in the soil and feed on roots while adults feed on above-ground parts of plants, but this seems to have little effect on plant growth. The major damage is caused by the female piercing plant tissues with her ovipositor to lay eggs. The cuts made by the three species of Amphipsalta form a herring-bone pattern (Fig. 2), and twigs and branches so affected may be sufficiently weakened to break in high winds. Such broken branches on conifers show up as reddish "flags" in the canopy when the foliage dies. Open cuts also provide entry for pathogens and wood- boring insects. Often the cuts heal over (Fig. 3) making the twigs gnarled in appearance. Ent_44_Amphipsalta_spp.doc Page 1 Fig. 2 - "Herring-bone" scars made by a female Amphipsalta when egglaying. The branch is of Eucalyptus ovata. Fig. 3 - Old egglaying damage. This Clerodendron twig has been cut to reveal calloused scar and ton egg deposition sites. Ent_44_Amphipsalta_spp.doc Page 2 Fig. 4 - Amphipsalta zelandica male above, female below. Hosts Amphipsalta females damage a wide range of native and exotic hardwood and softwood trees and shrubs. Distribution All cicadas in New Zealand are natives. Amphipsalta zelandica occurs throughout the country apart from central Otago and parts of Canterbury. -

An Appraisal of the Higher Classification of Cicadas (Hemiptera: Cicadoidea) with Special Reference to the Australian Fauna
© Copyright Australian Museum, 2005 Records of the Australian Museum (2005) Vol. 57: 375–446. ISSN 0067-1975 An Appraisal of the Higher Classification of Cicadas (Hemiptera: Cicadoidea) with Special Reference to the Australian Fauna M.S. MOULDS Australian Museum, 6 College Street, Sydney NSW 2010, Australia [email protected] ABSTRACT. The history of cicada family classification is reviewed and the current status of all previously proposed families and subfamilies summarized. All tribal rankings associated with the Australian fauna are similarly documented. A cladistic analysis of generic relationships has been used to test the validity of currently held views on family and subfamily groupings. The analysis has been based upon an exhaustive study of nymphal and adult morphology, including both external and internal adult structures, and the first comparative study of male and female internal reproductive systems is included. Only two families are justified, the Tettigarctidae and Cicadidae. The latter are here considered to comprise three subfamilies, the Cicadinae, Cicadettinae n.stat. (= Tibicininae auct.) and the Tettigadinae (encompassing the Tibicinini, Platypediidae and Tettigadidae). Of particular note is the transfer of Tibicina Amyot, the type genus of the subfamily Tibicininae, to the subfamily Tettigadinae. The subfamily Plautillinae (containing only the genus Plautilla) is now placed at tribal rank within the Cicadinae. The subtribe Ydiellaria is raised to tribal rank. The American genus Magicicada Davis, previously of the tribe Tibicinini, now falls within the Taphurini. Three new tribes are recognized within the Australian fauna, the Tamasini n.tribe to accommodate Tamasa Distant and Parnkalla Distant, Jassopsaltriini n.tribe to accommodate Jassopsaltria Ashton and Burbungini n.tribe to accommodate Burbunga Distant. -

Communication and Sexual Selection in the Barking Gecko(Ptenopus
Communication and Sexual Selection in the Barking Gecko (Ptenopus kochi) Daniel Adam Polakow Town Submittedfor fulfilment Capeofthe degree Master ofScience (MSc)of University Department ofZoology University of Cape To'!Il ' •I ,, " ;r ,.:;~,~.~~- r ' • • 'l•ob ' "'' , I I' ~" • 1 • 'l " f, .t ..h or. • (' - - .... ~ , Jo - - ;:. __ ...........,. ·~· ·~~ ·~ ·~, ·--..:. :.!-r:' . ..;c: -: .. ~ . ? ...~. ~·::. The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town To Joshua Julian, Inessa Triton, Peter, Apollo Russell, Lisa, Danielle, Pan and Nicolas - the most beautiful people in my world. ' Won 't you help to sing, these songs offreedom ... ' Bob Marley Redemption Song (1980) 11 Abstract This study focused on elucidating the functional significance of some aspects of the behaviour of Koch's barking gecko, Ptenopus kochi, during a field season conducted at the Desert Ecology Research Unit in Namibia for four months in 1995. Ptenopus kochi is a terrestrial species, and males were observed calling from their burrow entrances in the' dry Kuiseb river bed during the hot sununer months. First, aspects of competition among calling males were investigated. Calling males were seen to be non-randomly distributed relative to one another with evidence for regularity of spacing in dense aggregations. Sound intensity was investigated as the mechanism of spacing, and was mathematically modelled to gauge how the intensities of the calls of nearest-neighbour males overlapped. -

A New Species of Okanagana from the Walker Lane Region of Nevada and California (Hemiptera: Auchenorrhyncha: Cicadidae)
Zootaxa 4868 (4): 515–530 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4868.4.3 http://zoobank.org/urn:lsid:zoobank.org:pub:268A21D9-4AED-44C2-9236-38D632BC6ADA A new species of Okanagana from the Walker Lane region of Nevada and California (Hemiptera: Auchenorrhyncha: Cicadidae) WILL CHATFIELD-TAYLOR1 & JEFFREY A. COLE2,3,* 1Greenman-Pedersen Inc. �[email protected]; https://orcid.org/0000-0001-6509-4317 2Division of Natural Sciences, Pasadena City College, 1570 East Colorado Boulevard, Pasadena, CA 91106. �[email protected]; https://orcid.org/0000-0002-3485-6056 3Entomology Section, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, CA 90007 *Corresponding author Abstract Okanagana boweni sp. n. is described from the western margin of the Great Basin of North America. The new species is diagnosed from allopatric O. simulata Davis and sympatric O. utahensis Davis using morphological, bioacoustical, and molecular characters. The distribution of this new species coincides with the Walker Lane region that lies along the border of California and Nevada, USA. Based on geography, bioacoustics, morphology, and molecular phylogenetics, we hypothesize that O. boweni sp. n. is the allopatric sister species of O. simulata. Key words: bioacoustics, cicadas, allopatry, reproductive isolation, Lake Lahontan, Great Basin Introduction Of the 186 cicada species known in North America north of México (Sanborn & Heath 2017), 57 species (31%) belong to the genus Okanagana, with the majority of those species found in the western portion of the continent (Sanborn & Heath 2017; Sanborn & Phillips 2013).