Myxosporean Parasites in Australian Frogs and Tadpoles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular and Morphological Characterisation of The
Institute of Parasitology, Biology Centre CAS Folia Parasitologica 2021, 68: 007 doi: 10.14411/fp.2021.007 http://folia.paru.cas.cz Research Article Molecular and morphological characterisation of the metacercariae of two species of Cardiocephaloides (Digenea: Strigeidae) infecting endemic South African klipfish (Perciformes: Clinidae) Anja Vermaak1, Nico J. Smit1 and Olena Kudlai1,2,3 1 Water Research Group, Unit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa; 2 Institute of Ecology, Nature Research Centre, Vilnius, Lithuania; 3 Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic Abstract: South African clinids are a major component of the temperate intertidal regions that are also known to participate in life cycles and transmission of several groups of parasites. However, the knowledge of trematode diversity of these fishes is incomplete. In this study, two species of Clinus Cuvier, the super klipfish Clinus superciliosus (Linnaeus) and the bluntnose klipfish Clinus cot- toides Valenciennes, were collected from six localities along the South African coast and examined for the presence of trematodes. Metacercariae of Cardiocephaloides Sudarikov, 1959 were found in the eye vitreous humour and brain of C. superciliosus and in the eye vitreous humour of C. cottoides. Detailed analyses integrating morphological and molecular sequence data (28S rDNA, ITS2 rDNA-region, and COI mtDNA) revealed that these belong to two species, Cardiocephaloides physalis (Lutz, 1926) and an unknown species of Cardiocephaloides. This study provides the first report of clinid fishes serving as intermediate hosts for trematodes, reveals that the diversity of Cardiocephaloides in South Africa is higher than previously recorded, and highlights the need for further research to elucidate the life cycles of these trematode species. -

2.07 Frogs and Wetlands
Chapter 2.7 — Frogs and wetlands • 161 2.7 Frogs and wetlands Dr Arthur White Biosphere Environmental Consultants Pty Ltd Australia Abstract Australian frogs are remarkably diverse in their life styles and their use of wetlands. Our understanding of the ecological needs of frogs is incomplete but we do know some of the major requirements for survival, such as the need for clean water, the need for safe foraging areas, the need for shelter from predators and adverse weather conditions, the need for minimal habitat stress (as this increases the susceptibility of frogs to disease). The design of wetlands must take into account these over-riding requirements, plus the specific requirements that are unique to each frog species. In this paper, I refer to the management of the Green and Golden Bell Frog during the establishment of the Sydney Olympic site as an example of sorts of considerations that are required in managing frogs and wetlands. Chapter 2.7 — Frogs and wetlands • 162 Australian Frogs 2. Air pollution: massive amounts of Sulphur Dioxide and Nitrogen Dioxide (and There are about 250 described frog species in other gases) have been released into the Australia (Anstis 2013). This is a surprisingly high atmosphere since the Industrial Revolution. number of frog species for such an arid continent. These gases combine with moisture in the Australian frogs have had to adapt of the vagaries air and create toxic substances that kill frogs, of Australia’s climate and can survive in areas where other animals and plants. In the northern you would not expect them to be. -

Amphibia: Anura) Da Reserva Catuaba E Seu Entorno, Acre, Amazônia Sul-Ocidental
Efeitos da sucessão florestal sobre a anurofauna (Amphibia: Anura) da Reserva Catuaba e seu entorno, Acre, Amazônia sul-ocidental Vanessa M. de Souza 1; Moisés B. de Souza 2 & Elder F. Morato 2 1 Avenida Assis Chateaubriand 1170, ap. 304, Edifício Azul, Setor Oeste, 74130-011 Goiânia, Goiás, Brasil. E-mail: [email protected] 2 Departamento de Ciências da Natureza, Universidade Federal do Acre. Rodovia BR-364, km 04, Distrito Industrial, 69915-900 Rio Branco, Acre, Brasil. E-mail: [email protected]; [email protected] ABSTRACT. Effect of the forest succession on the anurans (Amphibia(Amphibia: Anura) of the Reserve Catuaba and its peripheryy, Acree, southwestern Amazonia. The objective of this work it was verify the abundance, richness, and the anuran composition in plots of vegetation of different succession stages in a forest and the matrix that surrounds it, of Acre (10º04’S, 67º37’W). The sampling was carried out between August 2005 and April 2006 in twelve plots located in three different sites in the forest. In each site four kinds of environments were chosen: primary forest (wood), secondary forest (capoeira), periphery (matrix) and secondary forest (succession). A total of twenty-seven species distributed in seven families was found. Greater abundance was registered in the plots of matrix two and capoeira three and the least in succession one. The richness was greater in matrix two, with the greater number of exclusive species. The abundance of anurans correlated significantly, with the average circum- ference at the breast height of the trees of the plots. The richness however correlated only marginally, with this structural feature. -

Sexual Maturity and Sexual Dimorphism in a Population of the Rocket-Frog Colostethus Aff
Tolosa et al. Actual Biol Volumen 37 / Número 102, 2014 Sexual maturity and sexual dimorphism in a population of the rocket-frog Colostethus aff. fraterdanieli (Anura: Dendrobatidae) on the northeastern Cordillera Central of Colombia Madurez y dimorfismo sexual de la ranita cohete Colostethus aff. fraterdanieli (Anura: Dendrobatidae) en una población al este de la Cordillera Central de Colombia Yeison Tolosa1, 2, *, Claudia Molina-Zuluaga1, 4,*, Adriana Restrepo1, 5, Juan M. Daza1, ** Abstract The minimum size of sexual maturity and sexual dimorphism are important life history traits useful to study and understand the population dynamics of any species. In this study, we determined the minimum size at sexual maturity and the existence of sexual dimorphism in a population of the rocket-frog, Colostethus aff. fraterdanieli, by means of morphological and morphometric data and macro and microscopic observation of the gonads. Females attained sexual maturity at 17.90 ± 0.1 mm snout-vent length (SVL), while males attained sexual maturity at 16.13 ± 0.06 mm SVL. Females differed from males in size, shape and throat coloration. Males were smaller than females and had a marked and dark throat coloration that sometimes extended to the chest, while females lacked this characteristic, with a throat either immaculate or weakly pigmented. In this study, we describe some important aspects of the reproductive ecology of a population of C. aff. fraterdanieli useful as a baseline for other more specialized studies. Key words: Amphibian, Andes, gonads, histology, morphometry, reproduction Resumen El tamaño mínimo de madurez sexual y el dimorfismo sexual son importantes características de historia de vida, útiles para estudiar y comprender la dinámica poblacional de cualquier especie. -

Breeding Activity of the Agile Frog Rana Dalmatina in a Rural Area M
Animal Biodiversity and Conservation 41.2 (2018) 405 Breeding activity of the agile frog Rana dalmatina in a rural area M. Biaggini, I. Campetti, C. Corti Biaggini, M., Campetti, I., Corti, C., 2018. Breeding activity of the agile frog Rana dalmatina in a rural area. Animal Biodiversity and Conservation, 41.2: 405–413, Doi: https://doi.org/10.32800/abc.2018.41.0405 Abstract Breeding activity of the agile frog Rana dalmatina in a rural area. Rural landscapes can host many protected species that are constantly threatened by agriculture intensification and abandonment of traditional manage- ments. Amphibians are severely affected by both processes due to loss and alteration of aquatic and terrestrial habitats. We monitored the breeding activity of Rana dalmatina in a lowland rural area focusing on spawning sites in open habitats, namely ditches amid traditional arable lands and pastures with varying vegetation fea- tures, size, and distances from woodlots. Egg clump density and clump size differed between sites, probably depending on environmental and ecological factors (i.e., larval competition, food availability, and predation). The sites next to woodlots showed the highest clump density (up to 0.718 n/m2). Our observations indicate that the maintenance and correct management of water bodies connected to traditional rural activities can be key to amphibian conservation in agricultural areas. Key words: Agriculture, Amphibians, Breeding sites, Conservation, Rana dalmatina Resumen Actividad reproductiva de la rana ágil Rana dalmatina en una zona rural. Los territorios agrícolas pueden ser refugio de varias especies protegidas que, sin embargo, están constantemente amenazadas por la intensificación de la agricultura y el abandono de las prácticas tradicionales. -
For Review Only
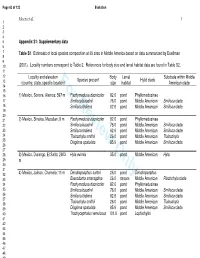
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

Species Diversity and Conservation Status of Amphibians in Madre De Dios, Southern Peru
Herpetological Conservation and Biology 4(1):14-29 Submitted: 18 December 2007; Accepted: 4 August 2008 SPECIES DIVERSITY AND CONSERVATION STATUS OF AMPHIBIANS IN MADRE DE DIOS, SOUTHERN PERU 1,2 3 4,5 RUDOLF VON MAY , KAREN SIU-TING , JENNIFER M. JACOBS , MARGARITA MEDINA- 3 6 3,7 1 MÜLLER , GIUSEPPE GAGLIARDI , LILY O. RODRÍGUEZ , AND MAUREEN A. DONNELLY 1 Department of Biological Sciences, Florida International University, 11200 SW 8th Street, OE-167, Miami, Florida 33199, USA 2 Corresponding author, e-mail: [email protected] 3 Departamento de Herpetología, Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Avenida Arenales 1256, Lima 11, Perú 4 Department of Biology, San Francisco State University, 1600 Holloway Avenue, San Francisco, California 94132, USA 5 Department of Entomology, California Academy of Sciences, 55 Music Concourse Drive, San Francisco, California 94118, USA 6 Departamento de Herpetología, Museo de Zoología de la Universidad Nacional de la Amazonía Peruana, Pebas 5ta cuadra, Iquitos, Perú 7 Programa de Desarrollo Rural Sostenible, Cooperación Técnica Alemana – GTZ, Calle Diecisiete 355, Lima 27, Perú ABSTRACT.—This study focuses on amphibian species diversity in the lowland Amazonian rainforest of southern Peru, and on the importance of protected and non-protected areas for maintaining amphibian assemblages in this region. We compared species lists from nine sites in the Madre de Dios region, five of which are in nationally recognized protected areas and four are outside the country’s protected area system. Los Amigos, occurring outside the protected area system, is the most species-rich locality included in our comparison. -

Interactions Between Amphibian Skin Sloughing and a Cutaneous Fungal Disease
Interactions between amphibian skin sloughing and a cutaneous fungal disease: infection progression, immune defence, and phylogenetic patterns Michel E. B. Ohmer BSc (Hons), MSc Zoology A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2016 School of Biological Sciences Abstract Worldwide, there has been an unprecedented rise in emerging infectious diseases of wildlife, and this has contributed to a widespread biodiversity crisis. Amphibian populations, in particular, are threatened by the fungal pathogen Batrachochytrium dendrobatidis (Bd), which in post-metamorphic animals only infects the skin, and causes the potentially lethal disease chytridiomycosis. Amphibians regularly slough their skin, and in doing so remove many skin- associated microbes. Thus, skin sloughing may play an important role in the pathogenesis of chytridiomycosis. To investigate this association, the influence of Bd infection on amphibian skin sloughing, and the role of sloughing in regulating infection, was examined. Furthermore, to better understand the variation in skin sloughing rates across species and ecological groups, and make inferences about the role of this process in susceptibility to this fungal disease, amphibian skin structure and function was investigated within a phylogenetic context. To determine the relationship between skin sloughing and disease progression (chapter 2), adult green tree frogs (Litoria caerulea) were exposed to an Australian Bd strain, and sloughing rates and infection load were monitored on a naturalistic cycling temperature regime (15 - 23˚C). Sloughing rates were determined by filming frogs and infection intensity was monitored before and after sloughing with conventional swabbing and quantitative PCR. Sloughing rate was found to increase with Bd infection load in infected frogs, but sloughing itself did not affect Bd load on the ventral skin surface. -

Bibliography and Scientific Name Index to Amphibians
lb BIBLIOGRAPHY AND SCIENTIFIC NAME INDEX TO AMPHIBIANS AND REPTILES IN THE PUBLICATIONS OF THE BIOLOGICAL SOCIETY OF WASHINGTON BULLETIN 1-8, 1918-1988 AND PROCEEDINGS 1-100, 1882-1987 fi pp ERNEST A. LINER Houma, Louisiana SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE NO. 92 1992 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. INTRODUCTION The present alphabetical listing by author (s) covers all papers bearing on herpetology that have appeared in Volume 1-100, 1882-1987, of the Proceedings of the Biological Society of Washington and the four numbers of the Bulletin series concerning reference to amphibians and reptiles. From Volume 1 through 82 (in part) , the articles were issued as separates with only the volume number, page numbers and year printed on each. Articles in Volume 82 (in part) through 89 were issued with volume number, article number, page numbers and year. -

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

THE AGILE FROG Species Action Plan Rana Dalmatina SUMMARSUMMARSUMMARYYY DOCUMENT
THE AGILE FROG Species Action Plan Rana dalmatina SUMMARSUMMARSUMMARYYY DOCUMENT The agile frog Rana dalmatina is Despite the efforts of these distributed widely throughout much of organisations, the future of Jersey’s southern and central Europe, but is agile frog is still far from secure. The found in only a few northern locations factors which probably played a key including Jersey - the frog is not found role in the frogs decline are still very anywhere else in the British Isles. The much in evidence: Jersey population of the agile frog has been declining in both range and •water quality and quantity, as a result SSSpppecial pointsss ofofof numbers since the early 1900’s. In the of intensive agriculture, are still below inininteresteresteresttt::: 1970’s only seven localities were listed EU standards in many areas; The agile frog is protected where the frog could still be found, and •the continuing alteration, disturbance, under schedule 1 of the by the mid 1980’s this had fallen to and loss of potentially suitable Conservation of Wildlife only two sites. In 1987 one of the amphibian habitat; (Jersey) Law 2000. remaining two populations was lost as a •the growing numbers of predatory result of a lethal spill of agricultural ducks, cats, and feral ferrets. DESCRIPTION pesticide into the breeding pond. The The agile frog Rana dalmatina species is now believed to be confined These and other factors have combined is a European brown frog, to a single vulnerable population in the to reduce the frog population to the growing up to 90 mm (snout south-west of the island. -

A New Species of Myxidium (Myxosporea: Myxidiidae)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln John Janovy Publications Papers in the Biological Sciences 6-2006 A New Species of Myxidium (Myxosporea: Myxidiidae), from the Western Chorus Frog, Pseudacris triseriata triseriata, and Blanchard's Cricket Frog, Acris crepitans blanchardi (Hylidae), from Eastern Nebraska: Morphology, Phylogeny, and Critical Comments on Amphibian Myxidium Taxonomy Miloslav Jirků University of Veterinary and Pharmaceutical Sciences, Palackého, [email protected] Matthew G. Bolek Oklahoma State University, [email protected] Christopher M. Whipps Oregon State University John J. Janovy Jr. University of Nebraska - Lincoln, [email protected] Mike L. Kent OrFollowegon this State and Univ additionalersity works at: https://digitalcommons.unl.edu/bioscijanovy Part of the Parasitology Commons See next page for additional authors Jirků, Miloslav; Bolek, Matthew G.; Whipps, Christopher M.; Janovy, John J. Jr.; Kent, Mike L.; and Modrý, David, "A New Species of Myxidium (Myxosporea: Myxidiidae), from the Western Chorus Frog, Pseudacris triseriata triseriata, and Blanchard's Cricket Frog, Acris crepitans blanchardi (Hylidae), from Eastern Nebraska: Morphology, Phylogeny, and Critical Comments on Amphibian Myxidium Taxonomy" (2006). John Janovy Publications. 60. https://digitalcommons.unl.edu/bioscijanovy/60 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in John Janovy Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Miloslav Jirků, Matthew G. Bolek, Christopher M. Whipps, John J. Janovy Jr., Mike L. Kent, and David Modrý This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ bioscijanovy/60 J.