Evaluation of Ecological Risk to Populations of a Threatened Plant from an Invasive Biocontrol Insect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Analysis and Implications of the Chloroplast Genomes of Three Thistles (Carduus L., Asteraceae)
Comparative analysis and implications of the chloroplast genomes of three thistles (Carduus L., Asteraceae) Joonhyung Jung1,*, Hoang Dang Khoa Do1,2,*, JongYoung Hyun1, Changkyun Kim1 and Joo-Hwan Kim1 1 Department of Life Science, Gachon University, Seongnam, Gyeonggi, Korea 2 Nguyen Tat Thanh Hi-Tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam * These authors contributed equally to this work. ABSTRACT Background. Carduus, commonly known as plumeless thistles, is a genus in the Asteraceae family that exhibits both medicinal value and invasive tendencies. However, the genomic data of Carduus (i.e., complete chloroplast genomes) have not been sequenced. Methods. We sequenced and assembled the chloroplast genome (cpDNA) sequences of three Carduus species using the Illumina Miseq sequencing system and Geneious Prime. Phylogenetic relationships between Carduus and related taxa were reconstructed using Maximum Likelihood and Bayesian Inference analyses. In addition, we used a single nucleotide polymorphism (SNP) in the protein coding region of the matK gene to develop molecular markers to distinguish C. crispus from C. acanthoides and C. tenuiflorus. Results. The cpDNA sequences of C. crispus, C. acanthoides, and C. tenuiflorus ranged from 152,342 bp to 152,617 bp in length. Comparative genomic analysis revealed high conservation in terms of gene content (including 80 protein-coding, 30 tRNA, and four rRNA genes) and gene order within the three focal species and members of subfamily Carduoideae. Despite their high similarity, the three species differed with respect to the number and content of repeats in the chloroplast genome. Additionally, eight Submitted 28 February 2020 hotspot regions, including psbI-trnS_GCU, trnE_UUC-rpoB, trnR_UCU-trnG_UCC, Accepted 11 December 2020 Published 14 January 2021 psbC-trnS_UGA, trnT_UGU-trnL_UAA, psbT-psbN, petD-rpoA, and rpl16-rps3, were identified in the study species. -

Biological Report
Biological Report 3093 Beachcomber Drive APN: 065-120-001 Morro Bay, CA Owner: Paul LaPlante Permit #29586 Prepared by V. L. Holland, Ph.D. Plant and Restoration Ecology 1697 El Cerrito Ct. San Luis Obispo, CA 93401 Prepared for: John K Construction, Inc. 110 Day Street Nipomo, CA 93444 [email protected] and Paul LaPlante 1935 Beachcomber Drive Morro Bay, CA 93442 March 5, 2013 BIOLOGICAL SURVEY OF 3093 BEACHCOMBER DRIVE, MORRO BAY, CA 2 TABLE OF CONTENTS EXECUTIVE SUMMARY ..................................................................................... 3 INTRODUCTION AND PURPOSE ...................................................................... 4 LOCATION AND PHYSICAL FEATURES ........................................................ 10 FLORISTIC, VEGETATION, AND WILDLIFE INVENTORY ............................. 11 METHODS ......................................................................................................... 11 RESULTS: FLORA AND VEGETATION ON SITE .......................................... 12 FLORA .............................................................................................................. 12 VEGETATION ..................................................................................................... 13 1. ANTHROPOGENIC (RUDERAL) COMMUNITIES ................................................... 13 2. COASTAL DUNE SCRUB ................................................................................. 15 SPECIAL STATUS PLANT SPECIES .............................................................. -

Thistles of Colorado
Thistles of Colorado About This Guide Identification and Management Guide Many individuals, organizations and agencies from throughout the state (acknowledgements on inside back cover) contributed ideas, content, photos, plant descriptions, management information and printing support toward the completion of this guide. Mountain thistle (Cirsium scopulorum) growing above timberline Casey Cisneros, Tim D’Amato and the Larimer County Department of Natural Resources Weed District collected, compiled and edited information, content and photos for this guide. Produced by the We welcome your comments, corrections, suggestions, and high Larimer County quality photos. If you would like to contribute to future editions, please contact the Larimer County Weed District at 970-498- Weed District 5769 or email [email protected] or [email protected]. Front cover photo of Cirsium eatonii var. hesperium by Janis Huggins Partners in Land Stewardship 2nd Edition 1 2 Table of Contents Introduction 4 Introduction Native Thistles (Pages 6-20) Barneyby’s Thistle (Cirsium barnebyi) 6 Cainville Thistle (Cirsium clacareum) 6 Native thistles are dispersed broadly Eaton’s Thistle (Cirsium eatonii) 8 across many Colorado ecosystems. Individual species occupy niches from Elk or Meadow Thistle (Cirsium scariosum) 8 3,500 feet to above timberline. These Flodman’s Thistle (Cirsium flodmanii) 10 plants are valuable to pollinators, seed Fringed or Fish Lake Thistle (Cirsium 10 feeders, browsing wildlife and to the centaureae or C. clavatum var. beauty and diversity of our native plant americanum) communities. Some non-native species Mountain Thistle (Cirsium scopulorum) 12 have become an invasive threat to New Mexico Thistle (Cirsium 12 agriculture and natural areas. For this reason, native and non-native thistles neomexicanum) alike are often pulled, mowed, clipped or Ousterhout’s or Aspen Thistle (Cirsium 14 sprayed indiscriminately. -

Supporting Analysis
APPENDIX A Supporting Analysis Table of Contents A.1 PARK SETTING ................................................................................................................................................ 2 A.2 DEMOGRAPHICS ............................................................................................................................................ 4 A.3 HISTORY OF THE LUDINGTON AREA ........................................................................................................... 6 A.4 HISTORY OF LUDINGTON STATE PARK ....................................................................................................... 7 A.5 LAND OWNERSHIP AND ACQUISITIONS ................................................................................................... 10 A.6 RELATIONSHIP TO OTHER RECREATION RESOURCES ............................................................................. 13 A.7 LEGAL MANDATES ........................................................................................................................................ 19 A.8 NATURAL SYSTEMS AND NATURAL RESOURCES ..................................................................................... 23 A.9 CULTURAL RESOURCES ............................................................................................................................... 27 A.10 EDUCATION AND INTERPRETATION ......................................................................................................... 30 A.11 RECREATION RESOURCES ......................................................................................................................... -

Mcgrath State Beach Plants 2/14/2005 7:53 PM Vascular Plants of Mcgrath State Beach, Ventura County, California by David L
Vascular Plants of McGrath State Beach, Ventura County, California By David L. Magney Scientific Name Common Name Habit Family Abronia maritima Red Sand-verbena PH Nyctaginaceae Abronia umbellata Beach Sand-verbena PH Nyctaginaceae Allenrolfea occidentalis Iodinebush S Chenopodiaceae Amaranthus albus * Prostrate Pigweed AH Amaranthaceae Amblyopappus pusillus Dwarf Coastweed PH Asteraceae Ambrosia chamissonis Beach-bur S Asteraceae Ambrosia psilostachya Western Ragweed PH Asteraceae Amsinckia spectabilis var. spectabilis Seaside Fiddleneck AH Boraginaceae Anagallis arvensis * Scarlet Pimpernel AH Primulaceae Anemopsis californica Yerba Mansa PH Saururaceae Apium graveolens * Wild Celery PH Apiaceae Artemisia biennis Biennial Wormwood BH Asteraceae Artemisia californica California Sagebrush S Asteraceae Artemisia douglasiana Douglas' Sagewort PH Asteraceae Artemisia dracunculus Wormwood PH Asteraceae Artemisia tridentata ssp. tridentata Big Sagebrush S Asteraceae Arundo donax * Giant Reed PG Poaceae Aster subulatus var. ligulatus Annual Water Aster AH Asteraceae Astragalus pycnostachyus ssp. lanosissimus Ventura Marsh Milkvetch PH Fabaceae Atriplex californica California Saltbush PH Chenopodiaceae Atriplex lentiformis ssp. breweri Big Saltbush S Chenopodiaceae Atriplex patula ssp. hastata Arrowleaf Saltbush AH Chenopodiaceae Atriplex patula Spear Saltbush AH Chenopodiaceae Atriplex semibaccata Australian Saltbush PH Chenopodiaceae Atriplex triangularis Spearscale AH Chenopodiaceae Avena barbata * Slender Oat AG Poaceae Avena fatua * Wild -

Checklist of the Vascular Plants of Redwood National Park
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 9-17-2018 Checklist of the Vascular Plants of Redwood National Park James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "Checklist of the Vascular Plants of Redwood National Park" (2018). Botanical Studies. 85. https://digitalcommons.humboldt.edu/botany_jps/85 This Flora of Northwest California-Checklists of Local Sites is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A CHECKLIST OF THE VASCULAR PLANTS OF THE REDWOOD NATIONAL & STATE PARKS James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State Univerity Arcata, California 14 September 2018 The Redwood National and State Parks are located in Del Norte and Humboldt counties in coastal northwestern California. The national park was F E R N S established in 1968. In 1994, a cooperative agreement with the California Department of Parks and Recreation added Del Norte Coast, Prairie Creek, Athyriaceae – Lady Fern Family and Jedediah Smith Redwoods state parks to form a single administrative Athyrium filix-femina var. cyclosporum • northwestern lady fern unit. Together they comprise about 133,000 acres (540 km2), including 37 miles of coast line. Almost half of the remaining old growth redwood forests Blechnaceae – Deer Fern Family are protected in these four parks. -
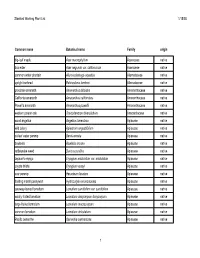
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -

Milk Thistle
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control BIOLOGY AND BIOLOGICAL CONTROL OF EXOTIC T RU E T HISTL E S RACHEL WINSTON , RICH HANSEN , MA R K SCH W A R ZLÄNDE R , ER IC COO M BS , CA R OL BELL RANDALL , AND RODNEY LY M FHTET-2007-05 U.S. Department Forest September 2008 of Agriculture Service FHTET he Forest Health Technology Enterprise Team (FHTET) was created in 1995 Tby the Deputy Chief for State and Private Forestry, USDA, Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ On the cover: Italian thistle. Photo: ©Saint Mary’s College of California. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at 202-720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, D.C. 20250-9410 or call 202-720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. The use of trade, firm, or corporation names in this publication is for information only and does not constitute an endorsement by the U.S. -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Recovery Strategy for Pitcher's Thistle (Cirsium Pitcheri) in Canada
Species at Risk Act Recovery Strategy for Pitcher’s Thistle (Cirsium pitcheri) in CanadaRECOVERY—June 2010 STRATEGY SERIES Recovery Strategy for Pitcher’s Thistle (Cirsium pitcheri) in Canada Pitcher’s Thistle 2011 i Recovery Strategy for Pitcher’s Thistle in Canada 2011 About the Species at Risk Act Recovery Strategy Series What is the Species at Risk Act (SARA)? SARA is the Act developed by the federal government as a key contribution to the common national effort to protect and conserve species at risk in Canada. SARA came into force in 2003 and one of its purposes is “to provide for the recovery of wildlife species that are extirpated, endangered or threatened as a result of human activity.” What is recovery? In the context of species at risk conservation, recovery is the process by which the decline of an endangered, threatened or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of the species’ persistence in the wild. A species will be considered recovered when its long-term persistence in the wild has been secured. What is a recovery strategy? A recovery strategy is a planning document that identifies what needs to be done to arrest or reverse the decline of a species. It sets goals and objectives and identifies the main areas of activities to be undertaken. Detailed planning is done at the action plan stage. Recovery strategy development is a commitment of all provinces and territories and of three federal agencies — Environment Canada, Parks Canada Agency and Fisheries and Oceans Canada — under the Accord for the Protection of Species at Risk. -

Spiranthes Diluvialis) and for Designated Weeds
2000 Survey of BLM-Managed Public Lands in Southwestern Wyoming for Ute Ladies Tresses (Spiranthes diluvialis) and for Designated Weeds Report Prepared for the BLM Rock Springs Field Office by George P. Jones, Wyoming Natural Diversity Database (University of Wyoming) in partial fulfillment of Cooperative Agreement K910A970018, Task Order TO-13 April 2001 TABLE OF CONTENTS ABSTRACT.........................................................................................................................i BACKGROUND................................................................................................................. 1 METHODS.......................................................................................................................... 1 RESULTS............................................................................................................................ 2 SPIRANTHES DILUVALIS .................................................................................. 2 WEEDS ................................................................................................................... 2 DISCUSSION ..................................................................................................................... 2 REFERENCES.................................................................................................................... 3 APPENDIX 1: DESCRIPTIONS OF STREAM SEGMENTS .........................................8 APPENDIX 2: ABUNDANCE OF THE DESIGNATED WEEDS IN EACH STREAM SEGMENT........................................................................................................................18 -

Onopordum Acanthium): Safe for Australia, but Not the USA?
Biological Control 41 (2007) 134–141 www.elsevier.com/locate/ybcon Lixus cardui, a biological control agent for scotch thistle (Onopordum acanthium): Safe for Australia, but not the USA? Joe Balciunas * USDA-ARS Exotic and Invasive Research Unit, Western Regional Research Center, 800 Buchanan St., Albany, CA 94710, USA Received 16 November 2006; accepted 19 December 2006 Available online 28 December 2006 Abstract Invasive exotic plants are often weeds in more than one country. After a biological control agent for a weed has been developed for use in one country, it is reasonable to consider using the same agent against the same weed in another country. ‘Transfer Projects’ can save considerable time and money, and they have been popular around the world. Lixus cardui Olivier (Coleoptera: Curculionidae), a weevil from Europe, was first used by Australian researchers to control Scotch thistle, Onopordum acanthium L. (Asteraceae). There are few close relatives of Scotch thistle in Australia, but that is not the case in North America, where scotch thistle is also an important weed. I initiated a project to test some of the agents released in Australia to see if they would be appropriate for release in the United States. Test plants, primarily Cirsium spp. thistles native to California, were exposed under both choice and no-choice conditions to two pop- ulations of L. cardui, one from Greece, the other from France. The latter may represent an undescribed species, and its test results are reported separately. Both strains of L. cardui weevils fed heavily and developed on some native North American thistles, at a level com- parable to the target weed, Scotch thistle.