Sensors and Regulators of Intracellular Ph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Assessing the Influence of Environmental Ph on Algal Physiology Using a Novel Culture System
Assessing the influence of environmental pH on algal physiology using a novel culture system By Rachel L. Golda A DISSERTATION Presented to the Division of Environmental and Biomolecular Systems and the Oregon Health & Science University School of Medicine in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Environmental Science and Engineering August, 2017 i School of Medicine Oregon Health & Science University CERTIFICATE OF APPROVAL _________________________________ This is to certify that the PhD dissertation of Rachel L. Golda has been approved ________________________________________________ Mentor/Advisor: Tawnya Peterson (Oregon Health & Science University) ________________________________________________ Mentor/Advisor: Joseph Needoba (Oregon Health & Science University) ________________________________________________ Committee Chair: Paul Tratnyek (Oregon Health & Science University) ________________________________________________ Member: Anne Thompson (Portland State University) i TABLE OF CONTENTS CERTIFICATE OF APPROVAL ........................................................... Error! Bookmark not defined. TABLE OF CONTENTS ........................................................................................................................... ii LIST OF FIGURES .................................................................................................................................... v LIST OF EQUATIONS ........................................................................................................................... -

THE ROLE of CARBON DIOXIDE (AND INTRACELLULAR Ph) IN
THEORETICAL ARTICLE—ELMÉLETI ÖSSZEFOGLALÁS THE ROLE OF CARBON DIOXIDE (AND INTRACELLULAR pH) IN THE PATHOMECHANISM OF SEVERAL MENTAL DISORDERS ARE THE DISEASES OF CIVILIZATION CAUSED BY LEARNT BEHAVIOUR, NOT THE STRESS ITSELF? ANDRÁS SIKTER , GÁBOR FALUDI , ZOLTÁN RIHMER 1 Municipal Clinic of Szentendre, Section of Internal Medicine 2 Dept of Clinical and Theoretical Mental Health, Kutvolgyi Clinical Center, Semmelweis University, Budapest ELMÉLETI ÖSSZEFOGLALÁS Neuropsychopharmacologia Hungarica 2009, XI/3, 161-173 A SZÉNDIOXID (ÉS AZ INTRACELLULÁRIS matikus betegségek patomechanizmusában. Fel- pH) SZEREPE NÉHÁNY MENTÁLIS tételezik, hogy a civilizációs betegségeket nem BETEGSÉG PATOMECHANIZMUSÁBAN maga a stressz, hanem annak le nem reagálása Nem maga a stressz, hanem tanult okozza azáltal, hogy a CO2 szint tartósan eltér a viselkedési formák okoznák a civilizációs fiziológiástól. A növekvõ agyi pCO2, acidotikus betegségeket? citoszol pH, és/vagy emelkedett bazális citoszol A széndioxid szerepe alábecsült a neuropszichi- Ca2+ koncentráció csökkenti a citoszolba történõ átriai betegségek patomechanizmusában, ugyan- Ca2+ beáramlást és az arousalt – dysthymiát, de- akkor fontos kapocs a lélek és a test között. A pressziót okozhatnak. Ez többnyire ATP hiány- mindenkori lelki állapot többnyire a légzést is nyal és a citoszol Mg2+ tartalmának csökkenésé- befolyásolja (lassítja, gyorsítja, irregulárissá te- vel is jár. Ez az energetikai és ionkonstelláció jel- szi), ezért változik a pH. Másrészt a neuronok lemzõ az életkor emelkedésével korrelációt mu- citoszoljának aktuális pH-ja a Ca2+ konduktivitás tató krónikus szervi betegségekre is, és a legfon- egyik legfontosabb modifikátora, ezért a légzés a tosabb kapcsolat az organikus betegségekkel, Ca2+-on keresztül közvetlenül, gyorsan, hatéko- például az iszkémiás szívbetegséggel. A felvá- nyan befolyásolja a “second messenger” rend- zolt modellbe beleillik, hogy egyes farmakológi- szert. -

ATP6V0A2 Rabbit Polyclonal Antibody – TA308549 | Origene
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TA308549 ATP6V0A2 Rabbit Polyclonal Antibody Product data: Product Type: Primary Antibodies Applications: WB Recommended Dilution: WB:1:1000-1:10000 Reactivity: Human, Mouse Host: Rabbit Isotype: IgG Clonality: Polyclonal Immunogen: Recombinant fragment corresponding to a region within amino acids 156 and 434 of ATP6V0A2 (Uniprot ID#Q9Y487) Formulation: 1XPBS, 20% Glycerol (pH7). 0.01% Thimerosal was added as a preservative. Concentration: lot specific Purification: Purified by antigen-affinity chromatography. Conjugation: Unconjugated Storage: Store at -20°C as received. Stability: Stable for 12 months from date of receipt. Predicted Protein Size: 98 kDa Gene Name: ATPase H+ transporting V0 subunit a2 Database Link: NP_036595 Entrez Gene 21871 MouseEntrez Gene 23545 Human Q9Y487 Background: The multisubunit vacuolar-type proton pump (H(+)-ATPase or V-ATPase) is essential for acidification of diverse cellular components, including endosomes, lysosomes, clathrin-coated vesicles, secretory vesicles, and chromaffin granules, and it is found at high density in the plasma membrane of certain specialized cells. H(+)-ATPases are comprised of a peripheral V(1) domain and an integral membrane V(0) domain; ATP6V0A2 is a component of the V(0) domain (Smith et al., 2003 [PubMed 14580332]). [supplied by OMIM] Synonyms: A2; ARCL; ARCL2A; ATP6A2; -

Genomic and Functional Profiling of Duplicated Chromosome
Human Molecular Genetics, 2006, Vol. 15, No. 6 853–869 doi:10.1093/hmg/ddl004 Advance Access published on January 30, 2006 Genomic and functional profiling of duplicated chromosome 15 cell lines reveal regulatory alterations in UBE3A-associated ubiquitin–proteasome pathway processes Colin A. Baron1, Clifford G. Tepper2, Stephenie Y. Liu1, Ryan R. Davis1, Nicholas J. Wang3, N. Carolyn Schanen4 and Jeffrey P. Gregg1,* 1Department of Pathology and 2Department of Biochemistry and Molecular Medicine, University of California, Davis School of Medicine, Sacramento, CA 95817, USA, 3Department of Human Genetics, University of 4 California–Los Angeles, Los Angeles, CA, USA and Center for Pediatric Research, Nemours Biomedical Research, Downloaded from Alfred I. duPont Hospital for Children, Wilmington, DE, USA Received November 21, 2005; Revised and Accepted January 25, 2006 Autism is a complex neurodevelopmental disorder having both genetic and epigenetic etiological elements. hmg.oxfordjournals.org Isodicentric chromosome 15 (Idic15), characterized by duplications of the multi-disorder critical region of 15q11–q14, is a relatively common cytogenetic event. When the duplication involves maternally derived con- tent, this abnormality is strongly correlated with autism disorder. However, the mechanistic links between Idic15 and autism are ill-defined. To gain insight into the potential role of these duplications, we performed a comprehensive, genomics-based characterization of an in vitro model system consisting of lymphoblast cell lines derived from individuals with both autism and Idic15. Array-based comparative genomic hybridiz- ation using commercial single nucleotide polymorphism arrays was conducted and found to be capable of by guest on December 17, 2010 sub-classifying Idic15 samples by virtue of the lengths of the duplicated chromosomal region. -

Does Aerobic Respiration Produce Carbon Dioxide Or Hydrogen Ion and Bicarbonate?
SPECIAL ARTICLE Does Aerobic Respiration Produce Carbon Dioxide or Hydrogen Ion and Bicarbonate? Erik R. Swenson, M.D. ABSTRACT Maintenance of intracellular pH is critical for clinical homeostasis. The metabolism of glucose, fatty acids, and amino acids yielding the generation of adenosine triphosphate in the mitochondria is accompanied by the production of acid in the Krebs Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/128/5/873/381029/20180500_0-00010.pdf by guest on 30 September 2021 cycle. Both the nature of this acidosis and the mechanism of its disposal have been argued by two investigators with a long- abiding interest in acid–base physiology. They offer different interpretations and views of the molecular mechanism of this intracellular pH regulation during normal metabolism. Dr. John Severinghaus has posited that hydrogen ion and bicarbon- ate are the direct end products in the Krebs cycle. In the late 1960s, he showed in brain and brain homogenate experiments that acetazolamide, a carbonic anhydrase inhibitor, reduces intracellular pH. This led him to conclude that hydrogen ion and bicarbonate are the end products, and the role of intracellular carbonic anhydrase is to rapidly generate diffusible carbon dioxide to minimize acidosis. Dr. Erik Swenson posits that carbon dioxide is a direct end product in the Krebs cycle, a more widely accepted view, and that acetazolamide prevents rapid intracellular bicarbonate formation, which can then codiffuse with carbon dioxide to the cell surface and there be reconverted for exit from the cell. Loss of this “facilitated diffusion of carbon dioxide” leads to intracellular acidosis as the still appreciable uncatalyzed rate of carbon dioxide hydration generates more protons. -

Nanomaterials for Intracellular Ph Sensing and Imaging
Nanomaterials for intracellular pH sensing and imaging Ying Lian, Wei Zhang, Longjiang Ding, Xiao-ai Zhang, Yinglu Zhang, Xu-dong Wang* Department of Chemistry, Fudan University, 200433, Shanghai, CHINA Western Chemistry bld. 114, Handan Road No. 220, Shanghai [email protected] Abstract: Intracellular pH is a vital parameter that precisely controls cell functionalities, activities and cellular events. Abnormal intracellular pH is always closely related to the healthy status of cells, which is further translated into pathological changes in a macro perspective. Because of the highly compartmentalized structure inside cells, the pH in each compartment can be precisely tuned to optimize certain cellular functionality, and biological reactions in these regions occur at optimum condition. Thus, it is important to design sensors that can precisely measure pH in these regions, and sensors must have good biocompatibility, physical stability, high sensitivity, wide measurement range, as well as fast response, to fulfill requirements for intracellular pH measurement. In this chapter, we will start from illustrating the importance of measuring intracellular pH, and further discuss how to design optical nanosensors for sensing and imaging intracellular pH. The state of the art technology in intracellular pH sensing and imaging will be reviewed, nanomaterials that are used for constructing intracellular pH sensors will be summarized and the perspective of nanomaterials for intracellular pH sensing and imaging will be given at the end. 1. Overview of the history of pH measurement pH is the abbreviation for Latin "Pondus hydrogenii", Pondus stands for power and hydrogenii stands for hydrogen. In chemistry, the pH scale is a numeric index used to specify acidity and alkalinity of aqueous solutions. -

New Insights Into the Physiological Role of Carbonic Anhydrase IX in Tumour Ph Regulation
Oncogene (2010) 29, 6509–6521 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 www.nature.com/onc REVIEW New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation P Swietach1, A Hulikova1, RD Vaughan-Jones1,3 and AL Harris2,3 1Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, UK and 2Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Oxford, UK In this review, we discuss the role of the tumour-associated acidity, commonly expressed using the pH scale. A carbonic anhydrase isoform IX (CAIX) in the context of particularly important class of pH-sensitive molecules is pH regulation. We summarise recent experimental find- protein because of the strong link between protonation ings on the effect of CAIX on cell growth and survival, and state, tertiary structure and functional output. The þ present a diffusion-reaction model to help in the assess- responsiveness to pH depends on H affinity (KH), ment of CAIX function under physiological conditions. bestowed on the protein by the chemistry of its amino CAIX emerges as an important facilitator of acid acid residues. For many proteins, KH is numerically diffusion and acid transport, helping to overcome large close to physiological [H þ ], thus even small displace- cell-to-capillary distances that are characteristic of solid ments from ‘normal’ intracellular pH (pHi) can sig- tumours. The source of substrate for CAIX catalysis is nificantly alter cellular biochemistry. H þ ions serve as likely to be CO2, generated by adequately oxygenated universal and potent modulators of virtually all aspects mitochondria or from the titration of metabolic acids with of life. -

The Role of the Golgi Apparatus in Disease (Review)
INTERNATIONAL JOURNAL OF MOleCular meDICine 47: 38, 2021 The role of the Golgi apparatus in disease (Review) JIANYANG LIU, YAN HUANG, TING LI, ZHENG JIANG, LIUWANG ZENG and ZHIPING HU Department of Neurology, Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, P.R. China Received August 12, 2020; Accepted January 15, 2021 DOI: 10.3892/ijmm.2021.4871 Abstract. The Golgi apparatus is known to underpin many 1. Introduction important cellular homeostatic functions, including trafficking, sorting and modifications of proteins or lipids. These functions The Golgi apparatus is a processing and sorting hub in the are dysregulated in neurodegenerative diseases, cancer, infec‑ transport and targeting of soluble cargo proteins and lipids to tious diseases and cardiovascular diseases, and the number of different destinations in the cell (1). Considering its central disease‑related genes associated with Golgi apparatus is on the role in the secretory pathway, alterations in the structure increase. Recently, many studies have suggested that the muta‑ and function of the Golgi apparatus are expected to affect tions in the genes encoding Golgi resident proteins can trigger the homeostasis of cellular proteins and lipids. Increasing the occurrence of diseases. By summarizing the pathogenesis of evidence suggests that structural changes and functional these genetic diseases, it was found that most of these diseases disorder of the Golgi apparatus are involved in many human have defects in membrane trafficking. Such defects typically diseases such as neurodegenerative diseases (2‑4), ischemic result in mislocalization of proteins, impaired glycosylation of stroke (5,6), cardiovascular diseases (7,8), pulmonary arterial proteins, and the accumulation of undegraded proteins. -
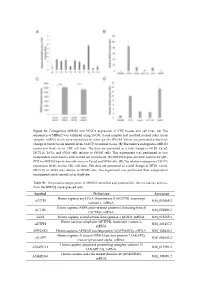
Figure S1. Endogenous MIR45
Figure S1. Endogenous MIR452 and VEGFA expression in CRC tissues and cell lines. (A) The expression of MIR452 was validated using 10 CRC tissue samples and matched normal colon tissue samples. miRNA levels were normalized to colon-specific RNU48. Values are presented as the fold- change in tumor tissue relative levels (ΔΔCT) to normal tissue. (B) The relative endogenous MIR452 expression levels in six CRC cell lines. The data are presented as a fold change in HT29, Caco2, HCT116, LoVo, and SW48 cells relative to SW480 cells. This experiment was performed as two independent experiments, each carried out in triplicate. (C) MIR452 expression level analysis by qRT- PCR for MIR452 transfection efficiency in Caco2 and SW48 cells. (D) The relative endogenous VEGFA expression levels in five CRC cell lines. The data are presented as a fold change in HT29, Caco2, HCT116, or SW48 cells relative to SW480 cells. This experiment was performed three independent experiments, each carried out in duplicate. Table S1. The putative target genes of MIR452 identified and predicted by the microarray analysis from the MIR452 overexpressed cells. Symbol Definition Accession Homo sapiens acyl-CoA thioesterase 8 (ACOT8), transcript ACOT8 NM_005469.2 variant 1, mRNA. Homo sapiens ARP6 actin-related protein 6 homolog (yeast) ACTR6 NM_022496.3 (ACTR6), mRNA. ADI1 Homo sapiens acireductone dioxygenase 1 (ADI1), mRNA. NM_018269.1 Homo sapiens aftiphilin (AFTPH), transcript variant 1, AFTPH NM_203437.2 mRNA. AHNAK2 Homo sapiens AHNAK nucleoprotein 2 (AHNAK2), mRNA. NM_138420.2 Homo sapiens A kinase (PRKA) anchor protein 7 (AKAP7), AKAP7 NM_004842.2 transcript variant alpha, mRNA. Homo sapiens anaphase promoting complex subunit 13 ANAPC13 NM_015391.2 (ANAPC13), mRNA. -

Autocrine IFN Signaling Inducing Profibrotic Fibroblast Responses By
Downloaded from http://www.jimmunol.org/ by guest on September 23, 2021 Inducing is online at: average * The Journal of Immunology , 11 of which you can access for free at: 2013; 191:2956-2966; Prepublished online 16 from submission to initial decision 4 weeks from acceptance to publication August 2013; doi: 10.4049/jimmunol.1300376 http://www.jimmunol.org/content/191/6/2956 A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Autocrine IFN Signaling Feng Fang, Kohtaro Ooka, Xiaoyong Sun, Ruchi Shah, Swati Bhattacharyya, Jun Wei and John Varga J Immunol cites 49 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2013/08/20/jimmunol.130037 6.DC1 This article http://www.jimmunol.org/content/191/6/2956.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 23, 2021. The Journal of Immunology A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Inducing Autocrine IFN Signaling Feng Fang,* Kohtaro Ooka,* Xiaoyong Sun,† Ruchi Shah,* Swati Bhattacharyya,* Jun Wei,* and John Varga* Activation of TLR3 by exogenous microbial ligands or endogenous injury-associated ligands leads to production of type I IFN. -

Supplemental Figures 04 12 2017
Jung et al. 1 SUPPLEMENTAL FIGURES 2 3 Supplemental Figure 1. Clinical relevance of natural product methyltransferases (NPMTs) in brain disorders. (A) 4 Table summarizing characteristics of 11 NPMTs using data derived from the TCGA GBM and Rembrandt datasets for 5 relative expression levels and survival. In addition, published studies of the 11 NPMTs are summarized. (B) The 1 Jung et al. 6 expression levels of 10 NPMTs in glioblastoma versus non‐tumor brain are displayed in a heatmap, ranked by 7 significance and expression levels. *, p<0.05; **, p<0.01; ***, p<0.001. 8 2 Jung et al. 9 10 Supplemental Figure 2. Anatomical distribution of methyltransferase and metabolic signatures within 11 glioblastomas. The Ivy GAP dataset was downloaded and interrogated by histological structure for NNMT, NAMPT, 12 DNMT mRNA expression and selected gene expression signatures. The results are displayed on a heatmap. The 13 sample size of each histological region as indicated on the figure. 14 3 Jung et al. 15 16 Supplemental Figure 3. Altered expression of nicotinamide and nicotinate metabolism‐related enzymes in 17 glioblastoma. (A) Heatmap (fold change of expression) of whole 25 enzymes in the KEGG nicotinate and 18 nicotinamide metabolism gene set were analyzed in indicated glioblastoma expression datasets with Oncomine. 4 Jung et al. 19 Color bar intensity indicates percentile of fold change in glioblastoma relative to normal brain. (B) Nicotinamide and 20 nicotinate and methionine salvage pathways are displayed with the relative expression levels in glioblastoma 21 specimens in the TCGA GBM dataset indicated. 22 5 Jung et al. 23 24 Supplementary Figure 4.