Targeting Oncogenic Signaling in Mutant FLT3 Acute Myeloid Leukemia: the Path to Least Resistance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Wu et al. Journal of Hematology & Oncology (2018) 11:133 https://doi.org/10.1186/s13045-018-0675-4 REVIEW Open Access FLT3 inhibitors in acute myeloid leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2* Abstract FLT3 mutations are one of the most common findings in acute myeloid leukemia (AML). FLT3 inhibitors have been in active clinical development. Midostaurin as the first-in-class FLT3 inhibitor has been approved for treatment of patients with FLT3-mutated AML. In this review, we summarized the preclinical and clinical studies on new FLT3 inhibitors, including sorafenib, lestaurtinib, sunitinib, tandutinib, quizartinib, midostaurin, gilteritinib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG 925, TTT-3002, and FF-10101. New generation FLT3 inhibitors and combination therapies may overcome resistance to first-generation agents. Keywords: FMS-like tyrosine kinase 3 inhibitors, Acute myeloid leukemia, Midostaurin, FLT3 Introduction RAS, MEK, and PI3K/AKT pathways [10], and ultim- Acute myeloid leukemia (AML) remains a highly resist- ately causes suppression of apoptosis and differentiation ant disease to conventional chemotherapy, with a me- of leukemic cells, including dysregulation of leukemic dian survival of only 4 months for relapsed and/or cell proliferation [11]. refractory disease [1]. Molecular profiling by PCR and Multiple FLT3 inhibitors are in clinical trials for treat- next-generation sequencing has revealed a variety of re- ing patients with FLT3/ITD-mutated AML. In this re- current gene mutations [2–4]. New agents are rapidly view, we summarized the preclinical and clinical studies emerging as targeted therapy for high-risk AML [5, 6]. on new FLT3 inhibitors, including sorafenib, lestaurtinib, In 1996, FMS-like tyrosine kinase 3/internal tandem du- sunitinib, tandutinib, quizartinib, midostaurin, gilteriti- plication (FLT3/ITD) was first recognized as a frequently nib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG mutated gene in AML [7]. -

Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling While Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells
Published OnlineFirst February 14, 2013; DOI: 10.1158/1535-7163.MCT-12-0305 Molecular Cancer Cancer Therapeutics Insights Therapeutics Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling while Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells Ruwanthi N. Gunawardane, Ronald R. Nepomuceno, Allison M. Rooks, Jeremy P. Hunt, Jill M. Ricono, Barbara Belli, and Robert C. Armstrong Abstract Fms-like tyrosine kinase 3 (FLT3) is implicated in the pathogenesis of acute myeloid leukemia (AML). FLT3-activating internal tandem duplication (ITD) mutations are found in approximately 30% of patients with AML and are associated with poor outcome in this patient population. Quizartinib (AC220) has previously been shown to be a potent and selective FLT3 inhibitor. In the current study, we expand on previous observations by showing that quizartinib potently inhibits the phosphorylation of FLT3 and downstream signaling molecules independent of FLT3 genotype, yet induces loss of viability only in cells expressing constitutively activated FLT3. We further show that transient exposure to quizartinib, whether in vitro or in vivo, leads to prolonged inhibition of FLT3 signaling, induction of apoptosis, and drastic reductions in tumor volume and pharmacodynamic endpoints. In vitro experiments suggest that these prolonged effects are mediated by slow binding kinetics that provide for durable inhibition of the kinase following drug removal/clearance. Together these data suggest quizartinib, with its unique combination of selectivity and potent/sustained inhibition of FLT3, may provide a safe and effective treatment against FLT3-driven leukemia. Mol Cancer Ther; 12(4); 1–10. Ó2013 AACR. Introduction downstream signaling cascades including the Ras/ Fms-like tyrosine kinase 3 (FLT3) is a member of the MAPK, PI3K/Akt, and STAT5 pathways (1, 9–13). -
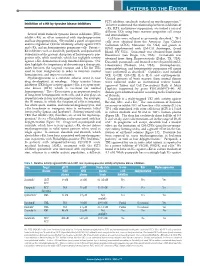
Letters to the Editor
LETTERS TO THE EDITOR FLT3 inhibitor, sorafenib, induced no myelosuppression.5,6 Inhibition of c-Kit by tyrosine kinase inhibitors To better understand the relationship between inhibition of c-Kit, FLT3, and marrow suppression, we studied a series of different TKIs using bone marrow progenitor cell assays Several small molecule tyrosine kinase inhibitors (TKIs) and immunoblots. inhibit c-Kit, an effect associated with myelosuppression Cell lines were cultured as previously described.2 TF-1 and hair depigmentation. We studied a panel of approved cells were obtained from the American Type Culture and investigational TKIs for inhibitory activity against FLT3 Collection (ATCC; Manassas, VA, USA) and grown in and c-Kit, and on hematopoietic progenitor cells. Potent c- RPMI supplemented with GM-CSF (Invitrogen, Grand Kit inhibitors such as dasatinib, pazopanib, and quizartinib Island, NY, USA). Quizartinib was obtained from Ambit demonstrated the greatest disruption of hematopoietic pro- Biosciences (San Diego, CA, USA). Crenolanib was genitor cells, while sorafenib, which has negligible activity obtained from Arog Pharmaceuticals (Dallas, TX, USA). against c-Kit, demonstrated only minimal disruption. Our Dasatinib, pazopanib, and imatinib were obtained from LC data highlight the importance of determining a therapeutic Laboratories (Woburn, MA, USA). Electrophoresis, index between the targeted receptor and c-Kit for TKIs immunoblotting, and hematopoietic progenitor cell assays used to treat malignancies in order to maintain normal were performed as described.2 Cytokines used included hematopoiesis and improve outcomes. SCF, G-CSF, GM-CSF, IL-3, IL-6, and erythropoietin. Myelosuppression is a common adverse event in new Unused portions of bone marrow from normal donors drug development in oncology. -

Isoliquiritigenin, an Orally Available Natural FLT3 Inhibitor from Licorice, Exhibits Selective Anti–Acute Myeloid Leukemia Efficacy in Vitro and in Vivo
1521-0111/96/5/589–599$35.00 https://doi.org/10.1124/mol.119.116129 MOLECULAR PHARMACOLOGY Mol Pharmacol 96:589–599, November 2019 Copyright ª 2019 by The American Society for Pharmacology and Experimental Therapeutics Isoliquiritigenin, an Orally Available Natural FLT3 Inhibitor from Licorice, Exhibits Selective Anti–Acute Myeloid Leukemia Efficacy In Vitro and In Vivo Zhi-Xing Cao,1 Yi Wen,1 Jun-Lin He, Shen-Zhen Huang, Fei Gao, Chuan-Jie Guo, Qing-Qing Liu, Shu-Wen Zheng, Dao-Yin Gong, Yu-Zhi Li, Ruo-Qi Zhang, Jian-Ping Chen, and Cheng Peng Pharmacy College, Chengdu University of Traditional Chinese Medicine, Ministry of Education Key Laboratory of Standardization Downloaded from of Chinese Herbal Medicine, Key Laboratory of Systematic Research, Development and Utilization of Chinese Medicine Resources in Sichuan Province-Key Laboratory Breeding Base of Co-founded by Sichuan Province and MOST, Chengdu, China (Z.-X.C., J.-L.H., C.-J.G., S.-W.Z., D.-Y.G., Y.-Z.L., R.-Q.Z., J.-P.C., C.P.);School of Chinese Medicine, University of Hong Kong, Hong Kong, China (Y.W., F.G., Q.-Q.L., J.-P.C.); College, Shenzhen Institute of Research and Innovation, University of Hong Kong, Shenzhen, China (Y.W., F.G., Q.-Q.L., J.-P.C.); and State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China (S.-Z.H.) molpharm.aspetjournals.org Received February 19, 2019; accepted August 20, 2019 ABSTRACT Licorice is a medicinal herb widely used to treat inflammation- AML cells. -

The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis Tsapogas, Panagiotis; Mooney, Ciaran; Brown, Geoffrey; Rolink, Antonius
University of Birmingham The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis Tsapogas, Panagiotis; Mooney, Ciaran; Brown, Geoffrey; Rolink, Antonius DOI: 10.3390/ijms18061115 License: Creative Commons: Attribution (CC BY) Document Version Publisher's PDF, also known as Version of record Citation for published version (Harvard): Tsapogas, P, Mooney, C, Brown, G & Rolink, A 2017, 'The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis', International Journal of Molecular Sciences, vol. 18, no. 6, pp. 1115. https://doi.org/10.3390/ijms18061115 Link to publication on Research at Birmingham portal General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. -

Crenolanib Is a Selective Type I Pan-FLT3 Inhibitor
Crenolanib is a selective type I pan-FLT3 inhibitor Catherine Choy Smitha,b,1, Elisabeth A. Lasatera,1, Kimberly C. Lina, Qi Wangc, Melissa Quino McCreeryd, Whitney K. Stewarta, Lauren E. Damona, Alexander E. Perle, Grace R. Jeschkee, Mayumi Sugitae, Martin Carrolle, Scott C. Koganb,d, John Kuriyanc, and Neil P. Shaha,b,2 aDivision of Hematology/Oncology, bHelen Diller Family Comprehensive Cancer Center, and dDivision of Laboratory Medicine, University of California, San Francisco, CA 94143; cDepartment of Molecular and Cell Biology, University of California, Berkeley, CA 94720; and eDivision of Hematology/Oncology, Perelman School of Medicine and the Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA 19104 Edited* by Charles L. Sawyers, Memorial Sloan–Kettering Cancer Center, New York, NY, and approved February 13, 2014 (received for review November 4, 2013) Tyrosine kinase inhibitors (TKIs) represent transformative thera- inhibitor and holds significant promise for achieving prolonged pies for several malignancies. Two critical features necessary for remissions. However, ponatinib potently inhibits a number of maximizing TKI tolerability and response duration are kinase selec- kinases, which may be responsible for unanticipated serious tivity and invulnerability to resistance-conferring kinase domain (KD) cardiovascular toxicities that have emerged with this agent. mutations in the intended target. No prior TKI has demonstrated The ability to selectively suppress resistance-conferring muta- both of these properties. -

A Phase 1/2 Study of the Oral FLT3 Inhibitor Pexidartinib in Relapsed/Refractory FLT3-ITD-Mutant Acute Myeloid Leukemia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Providence St. Joseph Health Digital Commons Providence St. Joseph Health Providence St. Joseph Health Digital Commons Articles, Abstracts, and Reports 4-28-2020 A phase 1/2 study of the oral FLT3 inhibitor pexidartinib in relapsed/refractory FLT3-ITD-mutant acute myeloid leukemia. Catherine C Smith Mark J Levis Olga Frankfurt John M Pagel SWEDISH CANCER INSTITUTE. Gail J Roboz See next page for additional authors Follow this and additional works at: https://digitalcommons.psjhealth.org/publications Part of the Hematology Commons, and the Oncology Commons Recommended Citation Smith, Catherine C; Levis, Mark J; Frankfurt, Olga; Pagel, John M; Roboz, Gail J; Stone, Richard M; Wang, Eunice S; Severson, Paul L; West, Brian L; Le, Mai H; Kayser, Sabine; Lam, Bao; Hsu, Henry H; Zhang, Chao; Bollag, Gideon; and Perl, Alexander E, "A phase 1/2 study of the oral FLT3 inhibitor pexidartinib in relapsed/refractory FLT3-ITD-mutant acute myeloid leukemia." (2020). Articles, Abstracts, and Reports. 3035. https://digitalcommons.psjhealth.org/publications/3035 This Article is brought to you for free and open access by Providence St. Joseph Health Digital Commons. It has been accepted for inclusion in Articles, Abstracts, and Reports by an authorized administrator of Providence St. Joseph Health Digital Commons. For more information, please contact [email protected]. Authors Catherine C Smith, Mark J Levis, Olga Frankfurt, John M Pagel, Gail J Roboz, Richard M Stone, Eunice S Wang, Paul L Severson, Brian L West, Mai H Le, Sabine Kayser, Bao Lam, Henry H Hsu, Chao Zhang, Gideon Bollag, and Alexander E Perl This article is available at Providence St. -

JULY 2019 Mrx Pipeline a View Into Upcoming Specialty and Traditional Drugs TABLE of CONTENTS
JULY 2019 MRx Pipeline A view into upcoming specialty and traditional drugs TABLE OF CONTENTS EDITORIAL STAFF Introduction Maryam Tabatabai, PharmD Editor in Chief Senior Director, Drug Information Pipeline Deep Dive Carole Kerzic, RPh Executive Editor Drug Information Pharmacist Keep on Your Radar Consultant Panel Michelle Booth, Pharm D Director, Medical Pharmacy Strategy Becky Borgert, PharmD, BCOP Pipeline Drug List Director, Clinical Oncology Product Development Lara Frick, PharmD, BCPS, BCPP Drug Information Pharmacist Glossary Robert Greer, RPh, BCOP Senior Director, Clinical Strategy and Programs YuQian Liu, PharmD Manager, Specialty Clinical Programs Troy Phelps Senior Director, Analytics Nothing herein is or shall be construed as a promise or representation regarding past or future events and Magellan Rx Management expressly disclaims any and all liability relating to the use of or reliance on the information contained in this presentation. The information contained in this publication is intended for educational purposes only and should not be considered clinical, financial, or legal advice. By receipt of this publication, each recipient agrees that the information contained herein will be kept confidential and that the information will not be photocopied, reproduced, distributed to, or disclosed to others at any time without the prior written consent of Magellan Rx Management. 1 | magellanrx.com INTRODUCTION Welcome to the MRx Pipeline. In its third year of publication, this quarterly report offers clinical insights and competitive intelligence on anticipated drugs in development. Our universal forecast addresses trends applicable across market segments. Traditional and specialty drugs, agents under the pharmacy and medical benefits, new molecular entities, pertinent new and expanded indications for existing medications, and biosimilars are profiled in the report. -

FLT3 Inhibitor, with Azacitidine (AZA) Or Low-Dose Cytarabine (LDAC) Might Improve the Outcomes in Patients with FLT3- ITD-Mutated AML
Acute Myeloid Leukemia ARTICLE A phase I/II study of the combination of quizartinib with azacitidine or low-dose Ferrata Storti Foundation cytarabine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome Mahesh Swaminathan, 1,2 Hagop M. Kantarjian, 1 Mark Levis, 3 Veronica Guerra, 1 Gautam Borthakur, 1 Yesid Alvarado, 1 Courtney D. DiNardo, 1 Tapan Kadia, 1 Guillermo Garcia-Manero, 1 Maro Ohanian, 1 Naval Daver, 1 Haematologica 2021 Marina Konopleva, 1 Naveen Pemmaraju, 1 Alessandra Ferrajol, 1 Volume 106(8):2121-2130 Michael Andreeff, 1 Nitin Jain, 1 Zeev Estrov, 1 Elias J. Jabbour, 1 William G. Wierda, 1 Sherry Pierce, 1 Maria Rhona Pinsoy, 1 Lianchun Xiao, 4 Farhad Ravandi 1 and Jorge E. Cortes 1,5 1Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX; 2Department of Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY; 3Department of Hematological Malignancies, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD; 4Department of Biostatistics, University of Texas MD Anderson Cancer Center, Houston, TX and 5Georgia Cancer Center at Augusta University, Augusta, GA, USA ABSTRACT he FMS-like tyrosine kinase 3-internal tandem duplication (F LT3- ITD) mutation in acute myeloid leukemia (AML) is associated with poor Tprognosis. We hypothesized that quizartinib, a selective and potent FLT3 inhibitor, with azacitidine (AZA) or low-dose cytarabine (LDAC) might improve the outcomes in patients with FLT3- ITD-mutated AML. In this open-label phase I/II trial, patients of any age receiving first-salvage treatment for FLT3- ITD AML or age >60 years with untreated myelodys- plastic syndrome or AML were treated with quizartinib plus AZA or LDAC. -

Clinical Benefits and Safety of FMS-Like Tyrosine Kinase 3
SYSTEMATIC REVIEW published: 03 June 2021 doi: 10.3389/fonc.2021.686013 Clinical Benefits and Safety of FMS-Like Tyrosine Kinase 3 Inhibitors in Various Treatment Stages of Acute Myeloid Leukemia: A Systematic Review, Meta-Analysis, and Network Meta-Analysis Edited by: Qingyu Xu 1,2, Shujiao He 1 and Li Yu 1* Adria´ n Mosquera Orgueira, University Hospital of Santiago 1 Department of Hematology and Oncology, International Cancer Center, Shenzhen Key Laboratory, Shenzhen University de Compostela, Spain General Hospital, Shenzhen University Clinical Medical Academy, Shenzhen University Health Science Center, 2 Reviewed by: Shenzhen, China, Department of Hematology and Oncology, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany Claudio Cerchione, Istituto Scientifico Romagnolo per lo Studio e il Trattamento dei Tumori Background: Given the controversial roles of FMS-like tyrosine kinase 3 inhibitors (FLT3i) (IRCCS), Italy Manuela Piazzi, in various treatment stages of acute myeloid leukemia (AML), this study was designed to National Research Council (CNR), assess this problem and further explored which FLT3i worked more effectively. Italy Bruno Quesnel, Methods: A systematic review, meta-analysis and network meta-analysis (NMA) were Centre Hospitalier Regional et conducted by filtering PubMed, Embase, Cochrane library, and Chinese databases. We Universitaire de Lille, France included studies comparing therapeutic effects between FLT3i and non-FLT3i group in *Correspondence: fi Li Yu AML, particularly FLT3(+) patients, or demonstrating the ef ciency of allogeneic [email protected] hematopoietic stem cell transplantation (allo-HSCT) in FLT3(+) AML. Relative risk (RR) with 95% confidence intervals (CI) was used for estimating complete remission (CR), early Specialty section: This article was submitted to death and toxicity. -

New Directions for Emerging Therapies in Acute Myeloid Leukemia: the Next Chapter Naval Daver 1,Andrewh.Wei2, Daniel A
Daver et al. Blood Cancer Journal (2020) 10:107 https://doi.org/10.1038/s41408-020-00376-1 Blood Cancer Journal REVIEW ARTICLE Open Access New directions for emerging therapies in acute myeloid leukemia: the next chapter Naval Daver 1,AndrewH.Wei2, Daniel A. Pollyea3,AmirT.Fathi4,PareshVyas 5 and Courtney D. DiNardo 1 Abstract Conventional therapy for acute myeloid leukemia is composed of remission induction with cytarabine- and anthracycline-containing regimens, followed by consolidation therapy, including allogeneic stem cell transplantation, to prolong remission. In recent years, there has been a significant shift toward the use of novel and effective, target- directed therapies, including inhibitors of mutant FMS-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase (IDH), the B-cell lymphoma 2 inhibitor venetoclax, and the hedgehog pathway inhibitor glasdegib. In older patients the combination of a hypomethylating agent or low-dose cytarabine, venetoclax achieved composite response rates that approximate those seen with standard induction regimens in similar populations, but with potentially less toxicity and early mortality. Preclinical data suggest synergy between venetoclax and FLT3- and IDH-targeted therapies, and doublets of venetoclax with inhibitors targeting these mutations have shown promising clinical activity in early stage trials. Triplet regimens involving the hypomethylating agent and venetoclax with FLT3 or IDH1/2 inhibitor, the TP53- modulating agent APR-246 and magrolimab, myeloid cell leukemia-1 inhibitors, or immune therapies such as CD123 antibody-drug conjugates and programmed cell death protein 1 inhibitors are currently being evaluated. It is hoped that such triplets, when applied in appropriate patient subsets, will further enhance remission rates, and more importantly remission durations and survival. -

Rare Diseases, When Taken Together, Are Are Together, When Taken Diseases, Rare to According at All
2013 MEDICINES IN DEVELOPMENT REPORT Rare Diseases A Report on Orphan Drugs in the Pipeline PRESENTED BY AMERICA’s biopharmACEUTICAL RESEARCH COMPANIES More Than 450 Medicines in Development for Rare Diseases Rare diseases, when taken together, are A major area of this research targets Orphan Drugs in Development* not that rare at all. In fact, according to rare cancers, accounting for more than Application the National Institutes of Health (NIH), one-third of all rare disease medicines in Submitted 30 million Americans have one of the development. Other top research areas Phase III nearly 7,000 diseases that are officially include genetic disorders, neurologi- Phase II deemed “rare” because alone they each cal conditions, infectious diseases and Phase I affect fewer than 200,000 people in autoimmune disorders. the United States. Sometimes, only Despite some recent victories, research a few hundred patients are known to 105 into treatments for rare diseases is a have a particular rare disease. daunting quest. This ongoing innovation Simply receiving a diagnosis of a rare and the hundreds of new medicines in disease often becomes a frustrating development now offer hope that physi- 85 quest, since many doctors may have nev- cians will have new treatment options er before heard of or seen the disease. for patients confronting a rare disease. This is, however, a time of great progress and hope. Biopharmaceutical 65 Contents research is entering an exciting new era Innovative Orphan Drugs with a growing understanding of the in the Pipeline ......................................... 2 human genome. Scientific advances have given researchers new tools to Orphan Drug Approvals ...........................4 explore rare diseases, which are often Challenges in Clinical Trials ......................6 more complex than common diseases.