FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Human Multi-Lineage Hepatic Organoid Model for Liver Fibrosis
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.01.278473; this version posted September 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A Human Multi-Lineage Hepatic Organoid Model for Liver Fibrosis Yuan Guan1, Annika Enejder2, Meiyue Wang1, Zhuoqing Fang1, Lu Cui3, Shih-Yu Chen4, Jingxiao Wang1, Yalun Tan1, Manhong Wu1, Xinyu Chen1, Patrik K. Johansson2, Issra Osman1, Koshi Kunimoto3, Pierre Russo5, Sarah C. Heilshorn2 and Gary Peltz1* 1Department of Anesthesia, Stanford University School of Medicine, Stanford CA, 94305; 2Department of Materials Science and Engineering, Stanford University, Stanford CA, 94305; 3Department of Pathology, Institute of Stem Cell Biology and Regenerative Medicine (ISCBRM), Stanford University School of Medicine, Stanford, 94305, CA, USA; 4Shih-Yu Chen, Institute of Biomedical Sciences, Academia Sinica, Taipei, 11529, Taiwan; 5Perelman School of Medicine at The University of Pennsylvania, Philadelphia, PA 19104 The authors have declared that no conflict of interest exists. *Address Correspondence to: [email protected] 300 Pasteur Dr. Room L232 Stanford, CA 94305. bioRxiv preprint doi: https://doi.org/10.1101/2020.09.01.278473; this version posted September 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Summary Despite its devastating consequences, liver fibrosis has no treatments. Genome engineering and a hepatic organoid system was used to produce the first in vitro model for human liver fibrosis. Hepatic organoids engineered to express the most common causative mutation for Autosomal Recessive Polycystic Kidney Disease (ARPKD) developed the key features of ARPKD liver pathology (abnormal bile ducts and hepatic fibrosis) in only 21 days. -

Childhood Leukemia
Onconurse.com Fact Sheet Childhood Leukemia The word leukemia literally means “white blood.” fungi. WBCs are produced and stored in the bone mar- Leukemia is the term used to describe cancer of the row and are released when needed by the body. If an blood-forming tissues known as bone marrow. This infection is present, the body produces extra WBCs. spongy material fills the long bones in the body and There are two main types of WBCs: produces blood cells. In leukemia, the bone marrow • Lymphocytes. There are two types that interact to factory creates an overabundance of diseased white prevent infection, fight viruses and fungi, and pro- cells that cannot perform their normal function of fight- vide immunity to disease: ing infection. As the bone marrow becomes packed with diseased white cells, production of red cells (which ° T cells attack infected cells, foreign tissue, carry oxygen and nutrients to body tissues) and and cancer cells. platelets (which help form clots to stop bleeding) slows B cells produce antibodies which destroy and stops. This results in a low red blood cell count ° foreign substances. (anemia) and a low platelet count (thrombocytopenia). • Granulocytes. There are four types that are the first Leukemia is a disease of the blood defense against infection: Blood is a vital liquid which supplies oxygen, food, hor- ° Monocytes are cells that contain enzymes that mones, and other necessary chemicals to all of the kill foreign bacteria. body’s cells. It also removes toxins and other waste products from the cells. Blood helps the lymph system ° Neutrophils are the most numerous WBCs to fight infection and carries the cells necessary for and are important in responding to foreign repairing injuries. -

Tyrosine Kinase Inhibitors Ameliorate Autoimmune Encephalomyelitis in a Mouse Model of Multiple Sclerosis
J Clin Immunol DOI 10.1007/s10875-011-9579-6 Tyrosine Kinase Inhibitors Ameliorate Autoimmune Encephalomyelitis in a Mouse Model of Multiple Sclerosis Oliver Crespo & Stacey C. Kang & Richard Daneman & Tamsin M. Lindstrom & Peggy P. Ho & Raymond A. Sobel & Lawrence Steinman & William H. Robinson Received: 23 February 2011 /Accepted: 5 July 2011 # Springer Science+Business Media, LLC 2011 Abstract Multiple sclerosis is an autoimmune disease of development of disease and treat established disease in a the central nervous system characterized by neuroinflam- mouse model of multiple sclerosis. In vitro, imatinib and mation and demyelination. Although considered a T cell- sorafenib inhibited astrocyte proliferation mediated by the mediated disease, multiple sclerosis involves the activation tyrosine kinase platelet-derived growth factor receptor of both adaptive and innate immune cells, as well as (PDGFR), whereas GW2580 and sorafenib inhibited resident cells of the central nervous system, which macrophage tumor necrosis factor (TNF) production medi- synergize in inducing inflammation and thereby demyelin- ated by the tyrosine kinases c-Fms and PDGFR, respec- ation. Differentiation, survival, and inflammatory functions tively. In vivo, amelioration of disease by GW2580 was of innate immune cells and of astrocytes of the central associated with a reduction in the proportion of macro- nervous system are regulated by tyrosine kinases. Here, we phages and T cells in the CNS infiltrate, as well as a show that imatinib, sorafenib, and GW2580—small mole- reduction in the levels of circulating TNF. Our findings cule tyrosine kinase inhibitors—can each prevent the suggest that GW2580 and the FDA-approved drugs imatinib and sorafenib have potential as novel therapeu- : : : tics for the treatment of autoimmune demyelinating O. -

Late Effects Among Long-Term Survivors of Childhood Acute Leukemia in the Netherlands: a Dutch Childhood Leukemia Study Group Report
0031-3998/95/3805-0802$03.00/0 PEDIATRIC RESEARCH Vol. 38, No.5, 1995 Copyright © 1995 International Pediatric Research Foundation, Inc. Printed in U.S.A. Late Effects among Long-Term Survivors of Childhood Acute Leukemia in The Netherlands: A Dutch Childhood Leukemia Study Group Report A. VAN DER DOES-VAN DEN BERG, G. A. M. DE VAAN, J. F. VAN WEERDEN, K. HAHLEN, M. VAN WEEL-SIPMAN, AND A. J. P. VEERMAN Dutch Childhood Leukemia Study Group,' The Hague, The Netherlands A.8STRAC ' Late events and side effects are reported in 392 children cured urogenital, or gastrointestinal tract diseases or an increased vul of leukemia. They originated from 1193 consecutively newly nerability of the musculoskeletal system was found. However, diagnosed children between 1972 and 1982, in first continuous prolonged follow-up is necessary to study the full-scale late complete remission for at least 6 y after diagnosis, and were effects of cytostatic treatment and radiotherapy administered treated according to Dutch Childhood Leukemia Study Group during childhood. (Pediatr Res 38: 802-807, 1995) protocols (70%) or institutional protocols (30%), all including cranial irradiation for CNS prophylaxis. Data on late events (relapses, death in complete remission, and second malignancies) Abbreviations were collected prospectively after treatment; late side effects ALL, acute lymphocytic leukemia were retrospectively collected by a questionnaire, completed by ANLL, acute nonlymphocytic leukemia the responsible pediatrician. The event-free survival of the 6-y CCR, continuous first complete remission survivors at 15 y after diagnosis was 92% (±2%). Eight late DCLSG, Dutch Childhood Leukemia Study Group relapses and nine second malignancies were diagnosed, two EFS, event free survival children died in first complete remission of late toxicity of HR, high risk treatment, and one child died in a car accident. -

Health | Childhood Cancer America's Children and the Environment
Health | Childhood Cancer Childhood Cancer Cancer is not a single disease, but includes a variety of malignancies in which abnormal cells divide in an uncontrolled manner. These cancer cells can invade nearby tissues and can migrate by way of the blood or lymph systems to other parts of the body.1 The most common childhood cancers are leukemias (cancers of the white blood cells) and cancers of the brain or central nervous system, which together account for more than half of new childhood cancer cases.2 Cancer in childhood is rare compared with cancer in adults, but still causes more deaths than any factor, other than injuries, among children from infancy to age 15 years.2 The annual incidence of childhood cancer has increased slightly over the last 30 years; however, mortality has declined significantly for many cancers due largely to improvements in treatment.2,3 Part of the increase in incidence may be explained by better diagnostic imaging or changing classification of tumors, specifically brain tumors.4 However, the President’s Cancer Panel recently concluded that the causes of the increased incidence of childhood cancers are not fully understood, and cannot be explained solely by the introduction of better diagnostic techniques. The Panel also concluded that genetics cannot account for this rapid change. The proportion of this increase caused by environmental factors has not yet been determined.5 The causes of cancer in children are poorly understood, though in general it is thought that different forms of cancer have different causes. According to scientists at the National Cancer Institute, established risk factors for the development of childhood cancer include family history, specific genetic syndromes (such as Down syndrome), high levels of radiation, and certain pharmaceutical agents used in chemotherapy.4,6 A number of studies suggest that environmental contaminants may play a role in the development of childhood cancers. -

Curing Childhood Leukemia, October 1997
This article was published in 1997 and has not been updated or revised. CURING CHILDHOOD LEUKEMIA ancer is an insidious disease. The culprit is not bacterial infections, viral infections, and many other a foreign invader, but the altered descendants illnesses. _) of our own cells, which reproduce uncontrol The fight against cancer has been more of a war of lably. In this civil war, it is hard to distinguish friend attrition than a series of spectacular, instantaneous vic from foe, to tat;get the cancer cells without killing the tories, and the research into childhood leukemia over the healthy cells. Most of our current cancer therapies, last 40 years is no exception. But most of the children who including the cure for childhood leukemia described here, are victims of this disease can now be cured, and the are based on the fact that cancer cells reproduce without drugs that made this possible are the antimetabolite some of the safeguards present in normal cells. If we can drugs that will be described here. The logic behind those interfere with cell reproduction, the cancer cells will be drugs came from a wide array of research that defined hit disproportionately hard and often will not recover. the chemical workings of the cell--research done by scien The scientists and physicians who devised the cure for tists who could not know that their findings would even childhood leukemia pioneered a rational approach to tually save the lives of up to thirty thousand children in destroying cancer cells, using knowledge about the cell the United States. -

07052020 MR ASCO20 Curtain Raiser
Media Release New data at the ASCO20 Virtual Scientific Program reflects Roche’s commitment to accelerating progress in cancer care First clinical data from tiragolumab, Roche’s novel anti-TIGIT cancer immunotherapy, in combination with Tecentriq® (atezolizumab) in patients with PD-L1-positive metastatic non- small cell lung cancer (NSCLC) Updated overall survival data for Alecensa® (alectinib), in people living with anaplastic lymphoma kinase (ALK)-positive metastatic NSCLC Key highlights to be shared on Roche’s ASCO virtual newsroom, 29 May 2020, 08:00 CEST Basel, 7 May 2020 - Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced that new data from clinical trials of 19 approved and investigational medicines across 21 cancer types, will be presented at the ASCO20 Virtual Scientific Program organised by the American Society of Clinical Oncology (ASCO), which will be held 29-31 May, 2020. A total of 120 abstracts that include a Roche medicine will be presented at this year's meeting. "At ASCO, we will present new data from many investigational and approved medicines across our broad oncology portfolio," said Levi Garraway, M.D., Ph.D., Roche's Chief Medical Officer and Head of Global Product Development. “These efforts exemplify our long-standing commitment to improving outcomes for people with cancer, even during these unprecedented times. By integrating our medicines and diagnostics together with advanced insights and novel platforms, Roche is uniquely positioned to deliver the healthcare solutions of the future." Together with its partners, Roche is pioneering a comprehensive approach to cancer care, combining new diagnostics and treatments with innovative, integrated data and access solutions for approved medicines that will both personalise and transform the outcomes of people affected by this deadly disease. -

Federal Register Notice 5-1-2020 Pdf Icon[PDF – 358
Federal Register / Vol. 85, No. 85 / Friday, May 1, 2020 / Notices 25439 confidential by the respondent (5 U.S.C. schedules. Other than examination DEPARTMENT OF HEALTH AND 552(b)(4)). reports, it provides the only financial HUMAN SERVICES Current actions: The Board has data available for these corporations. temporarily revised the instructions to The Federal Reserve is solely Centers for Disease Control and the FR Y–9C report to accurately reflect responsible for authorizing, supervising, Prevention the revised definition of ‘‘savings and assigning ratings to Edges. The [CDC–2020–0046; NIOSH–233–C] deposits’’ in accordance with the Federal Reserve uses the data collected amendments to Regulation D in the on the FR 2886b to identify present and Hazardous Drugs: Draft NIOSH List of interim final rule published on April 28, potential problems and monitor and Hazardous Drugs in Healthcare 2020 (85 FR 23445). Specifically, the develop a better understanding of Settings, 2020; Procedures; and Risk Board has temporarily revised the activities within the industry. Management Information instructions on the FR Y–9C, Schedule HC–E, items 1(b), 1(c), 2(c) and glossary Legal authorization and AGENCY: Centers for Disease Control and content to remove the transfer or confidentiality: Sections 25 and 25A of Prevention, HHS. withdrawal limit. As a result of the the Federal Reserve Act authorize the ACTION: Notice and request for comment. revision, if a depository institution Federal Reserve to collect the FR 2886b chooses to suspend enforcement of the (12 U.S.C. 602, 625). The obligation to SUMMARY: The National Institute for six transfer limit on a ‘‘savings deposit,’’ report this information is mandatory. -

Efficacy and Safety of Midostaurin-Based Induction and Maintenance Therapy for Newly Diagnosed AML
POST-ASH Issue 4, 2016 Efficacy and Safety of Midostaurin-Based Induction and Maintenance Therapy for Newly Diagnosed AML For more visit ResearchToPractice.com/5MJCASH2016 CME INFORMATION OVERVIEW OF ACTIVITY Each year, thousands of clinicians, basic scientists and other industry professionals sojourn to major international oncology conferences, like the American Society of Hematology (ASH) annual meeting, to hone their skills, network with colleagues and learn about recent advances altering state-of-the-art management in hematologic oncology. These events have become global stages where exciting science, cutting-edge concepts and practice-changing data emerge on a truly grand scale. This massive outpouring of information has enormous benefits for the hematologic oncology community, but the truth is it also creates a major challenge for practicing oncologists and hematologists. Although original data are consistently being presented and published, the flood of information unveiled during a major academic conference is unmatched and leaves in its wake an enormous volume of new knowledge that practicing oncologists must try to sift through, evaluate and consider applying. Unfortunately and quite commonly, time constraints and an inability to access these data sets leave many oncologists struggling to ensure that they’re aware of crucial practice-altering findings. This creates an almost insurmountable obstacle for clinicians in community practice because they are not only confronted almost overnight with thousands of new presentations and -

Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling While Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells
Published OnlineFirst February 14, 2013; DOI: 10.1158/1535-7163.MCT-12-0305 Molecular Cancer Cancer Therapeutics Insights Therapeutics Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling while Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells Ruwanthi N. Gunawardane, Ronald R. Nepomuceno, Allison M. Rooks, Jeremy P. Hunt, Jill M. Ricono, Barbara Belli, and Robert C. Armstrong Abstract Fms-like tyrosine kinase 3 (FLT3) is implicated in the pathogenesis of acute myeloid leukemia (AML). FLT3-activating internal tandem duplication (ITD) mutations are found in approximately 30% of patients with AML and are associated with poor outcome in this patient population. Quizartinib (AC220) has previously been shown to be a potent and selective FLT3 inhibitor. In the current study, we expand on previous observations by showing that quizartinib potently inhibits the phosphorylation of FLT3 and downstream signaling molecules independent of FLT3 genotype, yet induces loss of viability only in cells expressing constitutively activated FLT3. We further show that transient exposure to quizartinib, whether in vitro or in vivo, leads to prolonged inhibition of FLT3 signaling, induction of apoptosis, and drastic reductions in tumor volume and pharmacodynamic endpoints. In vitro experiments suggest that these prolonged effects are mediated by slow binding kinetics that provide for durable inhibition of the kinase following drug removal/clearance. Together these data suggest quizartinib, with its unique combination of selectivity and potent/sustained inhibition of FLT3, may provide a safe and effective treatment against FLT3-driven leukemia. Mol Cancer Ther; 12(4); 1–10. Ó2013 AACR. Introduction downstream signaling cascades including the Ras/ Fms-like tyrosine kinase 3 (FLT3) is a member of the MAPK, PI3K/Akt, and STAT5 pathways (1, 9–13). -
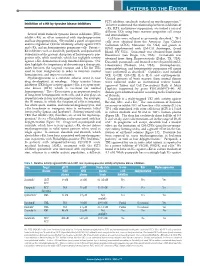
Letters to the Editor
LETTERS TO THE EDITOR FLT3 inhibitor, sorafenib, induced no myelosuppression.5,6 Inhibition of c-Kit by tyrosine kinase inhibitors To better understand the relationship between inhibition of c-Kit, FLT3, and marrow suppression, we studied a series of different TKIs using bone marrow progenitor cell assays Several small molecule tyrosine kinase inhibitors (TKIs) and immunoblots. inhibit c-Kit, an effect associated with myelosuppression Cell lines were cultured as previously described.2 TF-1 and hair depigmentation. We studied a panel of approved cells were obtained from the American Type Culture and investigational TKIs for inhibitory activity against FLT3 Collection (ATCC; Manassas, VA, USA) and grown in and c-Kit, and on hematopoietic progenitor cells. Potent c- RPMI supplemented with GM-CSF (Invitrogen, Grand Kit inhibitors such as dasatinib, pazopanib, and quizartinib Island, NY, USA). Quizartinib was obtained from Ambit demonstrated the greatest disruption of hematopoietic pro- Biosciences (San Diego, CA, USA). Crenolanib was genitor cells, while sorafenib, which has negligible activity obtained from Arog Pharmaceuticals (Dallas, TX, USA). against c-Kit, demonstrated only minimal disruption. Our Dasatinib, pazopanib, and imatinib were obtained from LC data highlight the importance of determining a therapeutic Laboratories (Woburn, MA, USA). Electrophoresis, index between the targeted receptor and c-Kit for TKIs immunoblotting, and hematopoietic progenitor cell assays used to treat malignancies in order to maintain normal were performed as described.2 Cytokines used included hematopoiesis and improve outcomes. SCF, G-CSF, GM-CSF, IL-3, IL-6, and erythropoietin. Myelosuppression is a common adverse event in new Unused portions of bone marrow from normal donors drug development in oncology. -

Disruption of CSF-1R Signaling Inhibits Growth of AML with Inv(16)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2021 Disruption of CSF-1R signaling inhibits growth of AML with inv(16) Simonis, Alexander ; Russkamp, Norman F ; Mueller, Jan ; Wilk, C Matthias ; Wildschut, Mattheus H E ; Myburgh, Renier ; Wildner-Verhey van Wijk, Nicole ; Mueller, Rouven ; Balabanov, Stefan ; Valk, Peter J M ; Theocharides, Alexandre P A ; Manz, Markus G DOI: https://doi.org/10.1182/bloodadvances.2020003125 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-202789 Journal Article Published Version The following work is licensed under a Publisher License. Originally published at: Simonis, Alexander; Russkamp, Norman F; Mueller, Jan; Wilk, C Matthias; Wildschut, Mattheus H E; Myburgh, Renier; Wildner-Verhey van Wijk, Nicole; Mueller, Rouven; Balabanov, Stefan; Valk, Peter J M; Theocharides, Alexandre P A; Manz, Markus G (2021). Disruption of CSF-1R signaling inhibits growth of AML with inv(16). Blood advances, 5(5):1273-1277. DOI: https://doi.org/10.1182/bloodadvances.2020003125 STIMULUS REPORT Disruption of CSF-1R signaling inhibits growth of AML with inv(16) Alexander Simonis,1,* Norman F. Russkamp,1,* Jan Mueller,1 C. Matthias Wilk,1 Mattheus H. E. Wildschut,1,2 Renier Myburgh,1 Nicole Wildner-Verhey van Wijk,1 Rouven Mueller,1 Stefan Balabanov,1 Peter J. M. Valk,3 Alexandre P. A. Theocharides,1 and Markus G. Manz1 1Department of Medical Oncology and Hematology, University Hospital