Specialty Pipeline Monthly Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FLT3 Inhibitors in Acute Myeloid Leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2*
Wu et al. Journal of Hematology & Oncology (2018) 11:133 https://doi.org/10.1186/s13045-018-0675-4 REVIEW Open Access FLT3 inhibitors in acute myeloid leukemia Mei Wu1, Chuntuan Li2 and Xiongpeng Zhu2* Abstract FLT3 mutations are one of the most common findings in acute myeloid leukemia (AML). FLT3 inhibitors have been in active clinical development. Midostaurin as the first-in-class FLT3 inhibitor has been approved for treatment of patients with FLT3-mutated AML. In this review, we summarized the preclinical and clinical studies on new FLT3 inhibitors, including sorafenib, lestaurtinib, sunitinib, tandutinib, quizartinib, midostaurin, gilteritinib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG 925, TTT-3002, and FF-10101. New generation FLT3 inhibitors and combination therapies may overcome resistance to first-generation agents. Keywords: FMS-like tyrosine kinase 3 inhibitors, Acute myeloid leukemia, Midostaurin, FLT3 Introduction RAS, MEK, and PI3K/AKT pathways [10], and ultim- Acute myeloid leukemia (AML) remains a highly resist- ately causes suppression of apoptosis and differentiation ant disease to conventional chemotherapy, with a me- of leukemic cells, including dysregulation of leukemic dian survival of only 4 months for relapsed and/or cell proliferation [11]. refractory disease [1]. Molecular profiling by PCR and Multiple FLT3 inhibitors are in clinical trials for treat- next-generation sequencing has revealed a variety of re- ing patients with FLT3/ITD-mutated AML. In this re- current gene mutations [2–4]. New agents are rapidly view, we summarized the preclinical and clinical studies emerging as targeted therapy for high-risk AML [5, 6]. on new FLT3 inhibitors, including sorafenib, lestaurtinib, In 1996, FMS-like tyrosine kinase 3/internal tandem du- sunitinib, tandutinib, quizartinib, midostaurin, gilteriti- plication (FLT3/ITD) was first recognized as a frequently nib, crenolanib, cabozantinib, Sel24-B489, G-749, AMG mutated gene in AML [7]. -

Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling While Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells
Published OnlineFirst February 14, 2013; DOI: 10.1158/1535-7163.MCT-12-0305 Molecular Cancer Cancer Therapeutics Insights Therapeutics Transient Exposure to Quizartinib Mediates Sustained Inhibition of FLT3 Signaling while Specifically Inducing Apoptosis in FLT3-Activated Leukemia Cells Ruwanthi N. Gunawardane, Ronald R. Nepomuceno, Allison M. Rooks, Jeremy P. Hunt, Jill M. Ricono, Barbara Belli, and Robert C. Armstrong Abstract Fms-like tyrosine kinase 3 (FLT3) is implicated in the pathogenesis of acute myeloid leukemia (AML). FLT3-activating internal tandem duplication (ITD) mutations are found in approximately 30% of patients with AML and are associated with poor outcome in this patient population. Quizartinib (AC220) has previously been shown to be a potent and selective FLT3 inhibitor. In the current study, we expand on previous observations by showing that quizartinib potently inhibits the phosphorylation of FLT3 and downstream signaling molecules independent of FLT3 genotype, yet induces loss of viability only in cells expressing constitutively activated FLT3. We further show that transient exposure to quizartinib, whether in vitro or in vivo, leads to prolonged inhibition of FLT3 signaling, induction of apoptosis, and drastic reductions in tumor volume and pharmacodynamic endpoints. In vitro experiments suggest that these prolonged effects are mediated by slow binding kinetics that provide for durable inhibition of the kinase following drug removal/clearance. Together these data suggest quizartinib, with its unique combination of selectivity and potent/sustained inhibition of FLT3, may provide a safe and effective treatment against FLT3-driven leukemia. Mol Cancer Ther; 12(4); 1–10. Ó2013 AACR. Introduction downstream signaling cascades including the Ras/ Fms-like tyrosine kinase 3 (FLT3) is a member of the MAPK, PI3K/Akt, and STAT5 pathways (1, 9–13). -
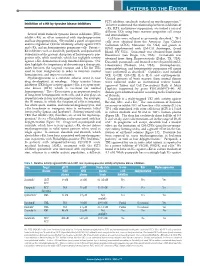
Letters to the Editor
LETTERS TO THE EDITOR FLT3 inhibitor, sorafenib, induced no myelosuppression.5,6 Inhibition of c-Kit by tyrosine kinase inhibitors To better understand the relationship between inhibition of c-Kit, FLT3, and marrow suppression, we studied a series of different TKIs using bone marrow progenitor cell assays Several small molecule tyrosine kinase inhibitors (TKIs) and immunoblots. inhibit c-Kit, an effect associated with myelosuppression Cell lines were cultured as previously described.2 TF-1 and hair depigmentation. We studied a panel of approved cells were obtained from the American Type Culture and investigational TKIs for inhibitory activity against FLT3 Collection (ATCC; Manassas, VA, USA) and grown in and c-Kit, and on hematopoietic progenitor cells. Potent c- RPMI supplemented with GM-CSF (Invitrogen, Grand Kit inhibitors such as dasatinib, pazopanib, and quizartinib Island, NY, USA). Quizartinib was obtained from Ambit demonstrated the greatest disruption of hematopoietic pro- Biosciences (San Diego, CA, USA). Crenolanib was genitor cells, while sorafenib, which has negligible activity obtained from Arog Pharmaceuticals (Dallas, TX, USA). against c-Kit, demonstrated only minimal disruption. Our Dasatinib, pazopanib, and imatinib were obtained from LC data highlight the importance of determining a therapeutic Laboratories (Woburn, MA, USA). Electrophoresis, index between the targeted receptor and c-Kit for TKIs immunoblotting, and hematopoietic progenitor cell assays used to treat malignancies in order to maintain normal were performed as described.2 Cytokines used included hematopoiesis and improve outcomes. SCF, G-CSF, GM-CSF, IL-3, IL-6, and erythropoietin. Myelosuppression is a common adverse event in new Unused portions of bone marrow from normal donors drug development in oncology. -

Nye Legemidler Som Ikke Har Markedsføringstillatelse for Legemidler Oppført På Listen Gjelder Retningslinjens Vilkår Uavhengig Av Indikasjon for Bruken
Nye legemidler som ikke har markedsføringstillatelse For legemidler oppført på listen gjelder retningslinjens vilkår uavhengig av indikasjon for bruken. Oppdatert: 13.11.2018 Orphan medicinal Indikasjon per nå. product (* Se (Samt mulig andre indikasjoner i informasjon Oppført på Virkestoff fremtiden.) nederst) listen Depatuximab mafodotin Treatment of glioblastoma (GBM) nov.18 Alpelisib Breast cancer; advanced hormone receptor positive (HR+), HER2-negative in men and postmenopausal women - second-line with fulvestrant okt.18 Omadacycline tosylate Treatment of community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure okt.18 Siponimod Treatmentinfections (ABSSSI) of secondary in adults progressive multiple sclerosis (SPMS) okt.18 autologous cd34+ cell enriched Treatment of transfusion-dependent β- population that contains thalassaemia (TDT) hematopoietic stem cells transduced with lentiglobin bb305 lentiviral vector encoding the beta-a-t87q-globin gene x okt.18 Fostamatinib Indicated for the treatment of thrombocytopenia okt.18 Dolutegravir / lamivudine Treatment of Human Immunodeficiency Virus type 1 (HIV-1) okt.18 Adeno-associated viral vector Treatment of paediatric patients serotype 9 containing the diagnosed with spinal muscular atrophy human SMN gene (AVXS-101) Type 1 okt.18 Treatment of non-neurological manifestations of acid sphingomyelinase Olipudase alfa deficiency okt.18 Treatment of adult and paediatric patients with locally advanced or Larotrectinib metastatic solid tumours x sep.18 Treatment -

F.No: QA/02/Central Inspection Plan/CT/Rdna/2018 Director
F.No: QA/02/Central Inspection Plan/CT/rDNA/2018 Director General of Health Services Central Drugs Standard Control Organisation Office of Drugs Controller General (India) FDA Bhawan, Kotla Road, New Delhi-110002 (QA Division) Dated: Office Memorandum As a process of Clinical trial oversight. CDSCO-HQ has prepared tentative Central Inspection Plan for the Year 2018 for inspections of the clinical trials permitted with respect tor-DNA products in accordance with the elements of SOP QA-INS-004. You are requested to conduct clinical trial inspections as per plan with the team comprising of inspectors trained in GCP. along with subject expert and an officer from CDSCO-HQ. Drugs inspectors from respecti ve states may also join the inspection team. The concerned COSCO Zonal officers are also requested to confirm the status of clinical trial site before planning the inspection at respective site. The clinical trial inspection report shall be submitted after completion of inspection to this office along with your recommendations for further action by this office. Drugs Controller G neral (India) Encl: 1. Central Inspection Plan for cl inical trial inspections for year 2018 for r-DNA products To: All DDC(l)s of North Zone, West Zone, South Zone, East Zone, Ahmedabad Zone, Hyderabad Zone. Varanasi Sub Zone, Jarnmu Sub Zone, Bangalore Sub zone and Baddi Sub Zone. Copy to: Guard fi le (QA Division & Biological division) PS to DCGI Central Drugs Standard Control Organization Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India FDA Bhavan, ITO, Kotla Road, New Delhi -110002 List of Clinical Trials (rDNA Products) to be inspected in the year 2018 Name of Investigational Sr. -

A Phase 3, Randomized, Double-Blind Study of Adjuvant Cemiplimab Versus Placebo Post-Surgery and Radiation in Patients With
433 A Phase 3, Randomized, Double-Blind Study of Adjuvant Cemiplimab Versus Placebo Post-Surgery and Radiation in Patients with High-Risk Cutaneous Squamous Cell Carcinoma (CSCC) Danny Rischin,1 Matthew G. Fury,2 Israel Lowy,2 Elizabeth Stankevich,3 Siyu Li,2 Hyunsil Han,2 Sandro V. Porceddu4 1Department of Medical Oncology, Peter MacCallum Cancer Centre, Melbourne, Australia; 2Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA; 3Regeneron Pharmaceuticals, Inc., Basking Ridge, NJ, USA; 4School of Medicine, University of Queensland, Herston, Queensland, Australia; Department of Radiation Oncology, Princess Alexandra Hospital, Woolloongabba, Queensland, Australia. Background Figure 1. C-POST study design Table 2. Key exclusion criteria • Squamous cell carcinoma arising from non-cutaneous sites Summary Cutaneous squamous cell carcinoma (CSCC) Patients with high-risk CSCC often experience relapse with Surgery, with high-risk • Concurrent malignancy other than localized CSCC and/or history of • CSCC is the second most common skin cancer with an estimated features on surgical locoregional recurrence or distant metastases despite initial malignancy other than localized CSCC within 3 years of date of • 1 pathology report incidence of around 1 million cases per year in the US. Worldwide, randomization, except for tumors with negligible risk of metastasis treatment with surgery and post-operative radiation. reports show an annual rise in incidence of 3–7% in most countries.2 350 mg cemiplimab Optional cemiplimab re-treatment after disease recurrence -

PRAC Draft Agenda of Meeting 4 -7 March 2013
4 March 2013 EMA/PRAC/141813/2013 Pharmacovigilance Risk Assessment Committee (PRAC) Pharmacovigilance Risk Assessment Committee (PRAC) Agenda of the meeting on 4-7 March 2013 Explanatory notes The Notes give a brief explanation of relevant agenda items and should be read in conjunction with the agenda. EU Referral procedures for safety reasons: Urgent EU procedures and Other EU referral procedures (Items 2 and 3 of the PRAC agenda) A referral is a procedure used to resolve issues such as concerns over the safety or benefit-risk balance of a medicine or a class of medicines. In a referral, the EMA is requested to conduct a scientific assessment of a particular medicine or class of medicines on behalf of the European Union (EU). For further detailed information on safety related referrals please see: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000150.jsp&mid =WC0b01ac05800240d0 Signals assessment and prioritisation (Item 4 of the PRAC agenda) A safety signal is information on a new or incompletely documented adverse event that is potentially caused by a medicine and that warrants further investigation. Signals are generated from several sources such as spontaneous reports, clinical studies and the scientific literature. The evaluation of safety signals is a routine part of pharmacovigilance and is essential to ensuring that regulatory authorities have a comprehensive knowledge of a medicine’s benefits and risks. The presence of a safety signal does not mean that a medicine has caused the reported adverse event. The adverse event could be a symptom of another illness or caused by another medicine taken by the patient. -

Isoliquiritigenin, an Orally Available Natural FLT3 Inhibitor from Licorice, Exhibits Selective Anti–Acute Myeloid Leukemia Efficacy in Vitro and in Vivo
1521-0111/96/5/589–599$35.00 https://doi.org/10.1124/mol.119.116129 MOLECULAR PHARMACOLOGY Mol Pharmacol 96:589–599, November 2019 Copyright ª 2019 by The American Society for Pharmacology and Experimental Therapeutics Isoliquiritigenin, an Orally Available Natural FLT3 Inhibitor from Licorice, Exhibits Selective Anti–Acute Myeloid Leukemia Efficacy In Vitro and In Vivo Zhi-Xing Cao,1 Yi Wen,1 Jun-Lin He, Shen-Zhen Huang, Fei Gao, Chuan-Jie Guo, Qing-Qing Liu, Shu-Wen Zheng, Dao-Yin Gong, Yu-Zhi Li, Ruo-Qi Zhang, Jian-Ping Chen, and Cheng Peng Pharmacy College, Chengdu University of Traditional Chinese Medicine, Ministry of Education Key Laboratory of Standardization Downloaded from of Chinese Herbal Medicine, Key Laboratory of Systematic Research, Development and Utilization of Chinese Medicine Resources in Sichuan Province-Key Laboratory Breeding Base of Co-founded by Sichuan Province and MOST, Chengdu, China (Z.-X.C., J.-L.H., C.-J.G., S.-W.Z., D.-Y.G., Y.-Z.L., R.-Q.Z., J.-P.C., C.P.);School of Chinese Medicine, University of Hong Kong, Hong Kong, China (Y.W., F.G., Q.-Q.L., J.-P.C.); College, Shenzhen Institute of Research and Innovation, University of Hong Kong, Shenzhen, China (Y.W., F.G., Q.-Q.L., J.-P.C.); and State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China (S.-Z.H.) molpharm.aspetjournals.org Received February 19, 2019; accepted August 20, 2019 ABSTRACT Licorice is a medicinal herb widely used to treat inflammation- AML cells. -

Libtayo® (Cemiplimab-Rwlc)
Libtayo® (cemiplimab-rwlc) (Intravenous) Document Number: IC-0398 Last Review Date: 06/01/2021 Date of Origin: 10/30/2018 Dates Reviewed: 11/2018, 03/2019, 06/2019, 09/2019, 12/2019, 03/2020, 6/2020, 12/2020, 03/2021, 06/2021 I. Length of Authorization Coverage will be provided for six months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: • Libtayo 350 mg/7 mL single-use vial: 1 vial per 21 days B. Max Units (per dose and over time) [HCPCS Unit]: • 350 billable units every 21 days III. Initial Approval Criteria1 Coverage is provided for the following conditions: • Patient is at least 18 years of age; AND Universal Criteria 1 • Patient has not received previous therapy with a programmed death (PD-1/PD-L1)-directed therapy (e.g., avelumab, pembrolizumab, atezolizumab, durvalumab, nivolumab, dostarlimab, etc.), unless otherwise specified; AND • Used as a single-agent therapy; AND • Patient has not received previous therapy with a cytotoxic T-lymphocyte antigen 4 (CTLA-4) targeting agent (e.g., ipilimumab, etc.) within the 4 weeks prior to therapy; AND Cutaneous Squamous Cell Carcinoma (CSCC) † 1-5 • Patient has nodal or distant metastatic disease, locally advanced disease, inoperable or not fully resectable regional disease, or regional recurrence; AND • Patient is not a candidate for curative surgery or curative radiation therapy Basal Cell Carcinoma (BCC) † 1,2,6 • Patient has locally advanced OR nodal, regional, or distant metastatic disease; AND Proprietary & Confidential © 2021 Magellan Health, -

Justification
Justification to the Resolution of the Federal Joint Committee (G-BA) on an Amendment of the Pharmaceuticals Directive (AM ‑ RL): Annex XII – Resolutions on the Benefit Assessment of Medicinal Products with New Active Ingredients in Accordance with Section 35a SGB V Damoctocog alfa pegol From 20 June 2019 Contents 1. Legal basis ................................................................................................................ 2 2. Key points of the resolution ..................................................................................... 2 2.1 Additional benefit of the medicinal product in relation to the appropriate comparator therapy ..................................................................................................... 3 2.1.1 Approved therapeutic indication of damoctocog alfa pegol (Jivi®) in accordance with the summary of product characteristics ............................................ 3 2.1.2 Appropriate comparator therapy ................................................................... 3 2.1.3 Extent and probability of the additional benefit .............................................. 5 2.1.4 Summary of the assessment ........................................................................ 6 2.2 Number of patients or demarcation of patient groups eligible for treatment ...... 6 2.3 Requirements for a quality-assured application ................................................ 7 2.4 Treatment costs .............................................................................................. -

The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis Tsapogas, Panagiotis; Mooney, Ciaran; Brown, Geoffrey; Rolink, Antonius
University of Birmingham The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis Tsapogas, Panagiotis; Mooney, Ciaran; Brown, Geoffrey; Rolink, Antonius DOI: 10.3390/ijms18061115 License: Creative Commons: Attribution (CC BY) Document Version Publisher's PDF, also known as Version of record Citation for published version (Harvard): Tsapogas, P, Mooney, C, Brown, G & Rolink, A 2017, 'The Cytokine Flt3-Ligand in Normal and Malignant Hematopoiesis', International Journal of Molecular Sciences, vol. 18, no. 6, pp. 1115. https://doi.org/10.3390/ijms18061115 Link to publication on Research at Birmingham portal General rights Unless a licence is specified above, all rights (including copyright and moral rights) in this document are retained by the authors and/or the copyright holders. The express permission of the copyright holder must be obtained for any use of this material other than for purposes permitted by law. •Users may freely distribute the URL that is used to identify this publication. •Users may download and/or print one copy of the publication from the University of Birmingham research portal for the purpose of private study or non-commercial research. •User may use extracts from the document in line with the concept of ‘fair dealing’ under the Copyright, Designs and Patents Act 1988 (?) •Users may not further distribute the material nor use it for the purposes of commercial gain. Where a licence is displayed above, please note the terms and conditions of the licence govern your use of this document. When citing, please reference the published version. Take down policy While the University of Birmingham exercises care and attention in making items available there are rare occasions when an item has been uploaded in error or has been deemed to be commercially or otherwise sensitive. -

Northwest Medical Benefit Formulary (List of Covered Drugs) Please Read
Northwest Medical Benefit Formulary (List of Covered Drugs) Please Read: This document contains information about the drugs we cover when they are administered to you in a Participating Medical Office. What is the Kaiser Permanente Northwest Medical Benefit Formulary? A formulary is a list of covered drugs chosen by a group of Kaiser Permanente physicians and pharmacists known as the Formulary and Therapeutics Committee. This committee meets regularly to evaluate and select the safest, most effective medications for our members. Kaiser Permanente Formulary The formulary list that begins on the next page provides information about some of the drugs covered by our plan when they are administered to you in a Participating Medical Office. Depending on your medical benefits, you may pay a cost share for the drug itself. The first column of the chart lists the drug’s generic name. The second column lists the brand name. Most administered medications and vaccines are only available as brand name drugs. Contraceptive Drugs and Devices Your provider may prescribe as medically necessary any FDA-approved contraceptive drug or device, including those on this formulary, which you will receive at no cost share. Last Updated 09/01/2021 Generic Name Brand Name Clinic Administered Medications (ADMD) ABATACEPT ORENCIA ABOBOTULINUM TOXIN A DYSPORT ADALIMUMAB HUMIRA ADO-TRASTUZUMAB EMTANSINE KADCYLA AFAMELANOTIDE ACETATE SCENESSE AFLIBERCEPT EYLEA AGALSIDASE BETA FABRAZYME ALDESLEUKIN PROLEUKIN ALEMTUZUMAB LEMTRADA ALGLUCOSIDASE ALFA LUMIZYME ARALAST, GLASSIA,