Congenital Cleft Sternum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Part 1 the Thorax ECA1 7/18/06 6:30 PM Page 2 ECA1 7/18/06 6:30 PM Page 3
ECA1 7/18/06 6:30 PM Page 1 Part 1 The Thorax ECA1 7/18/06 6:30 PM Page 2 ECA1 7/18/06 6:30 PM Page 3 Surface anatomy and surface markings The experienced clinician spends much of his working life relating the surface anatomy of his patients to their deep structures (Fig. 1; see also Figs. 11 and 22). The following bony prominences can usually be palpated in the living subject (corresponding vertebral levels are given in brackets): •◊◊superior angle of the scapula (T2); •◊◊upper border of the manubrium sterni, the suprasternal notch (T2/3); •◊◊spine of the scapula (T3); •◊◊sternal angle (of Louis) — the transverse ridge at the manubrio-sternal junction (T4/5); •◊◊inferior angle of scapula (T8); •◊◊xiphisternal joint (T9); •◊◊lowest part of costal margin—10th rib (the subcostal line passes through L3). Note from Fig. 1 that the manubrium corresponds to the 3rd and 4th thoracic vertebrae and overlies the aortic arch, and that the sternum corre- sponds to the 5th to 8th vertebrae and neatly overlies the heart. Since the 1st and 12th ribs are difficult to feel, the ribs should be enu- merated from the 2nd costal cartilage, which articulates with the sternum at the angle of Louis. The spinous processes of all the thoracic vertebrae can be palpated in the midline posteriorly, but it should be remembered that the first spinous process that can be felt is that of C7 (the vertebra prominens). The position of the nipple varies considerably in the female, but in the male it usually lies in the 4th intercostal space about 4in (10cm) from the midline. -

Costochondritis
Department of Rehabilitation Services Physical Therapy Standard of Care: Costochondritis Case Type / Diagnosis: Costochondritis ICD-9: 756.3 (rib-sternum anomaly) 727.2 (unspecified disorder of synovium) Costochondritis (CC) is a benign inflammatory condition of the costochondral or costosternal joints that causes localized pain. 1 The onset is insidious, though patient may note particular activity that exacerbates it. The etiology is not clear, but it is most likely related to repetitive trauma. Symptoms include intermittent pain at costosternal joints and tenderness to palpation. It most frequently occurs unilaterally at ribs 2-5, but can occur at other levels as well. Symptoms can be exacerbated by trunk movement and deep breathing, but will decrease with quiet breathing and rest. 2 CC usually responds to conservative treatment, including non-steroidal anti-inflammatory medication. A review of the relevant anatomy may be helpful in understanding the pathology. The chest wall is made up of the ribs, which connect the vertebrae posteriorly with the sternum anteriorly. Posteriorly, the twelve ribs articulate with the spine through both the costovertebral and costotransverse joints forming the most hypomobile region of the spine. Anteriorly, ribs 1-7 articulate with the costocartilages at the costochondral joints, which are synchondroses without ligamentous support. The costocartilage then attaches directly to the sternum as the costosternal joints, which are synovial joints having a capsule and ligamentous support. Ribs 8-10 attach to the sternum via the cartilage at the rib above, while ribs 11 and 12 are floating ribs, without an anterior articulation. 3 There are many causes of musculo-skeletal chest pain arising from the ribs and their articulations, including rib trauma, slipping rib syndrome, costovertebral arthritis and Tietze’s syndrome. -

Skeleton of the Spine and the Thorax
SKELETON OF THE SPINE AND THE THORAX Pages 37- 42 and 54 - 57 Skeleton of the spine Vertebral Column . forms the basic structure of the trunk . consists of 33-34 vertebrae and intervertebral discs . 7 cervical, 12 thoracic, 5 lumbar = true vertebrae . sacrum and coccyx fused = false vertebrae Vertebra . all vertebrae have certain features in common (vertebral body, vertebral arch and seven processes) and regional differences . vertebral body . vetrebral arch pedicle lamina spinous process transverse process articular processes . vertebral foramen . vetrebral notch Cervical vertebrae . transverse foramen (foramen transversarium) in the transverse process . transverse processes of cervical vertebrae end laterally in two projection for attachment of cervical muscles anterior tubercle and posterior tubercle . bifid spinous process . C6 - tuberculum caroticum . C7 - vertebra prominens Atlas C1 . a ring-shaped bone . has neither a boby nor a spinous process . lateral masses . anterior and posterior arches . anterior and posterior tubercles . superior and inferior articular surfaces . articular facet for dens Axis C2 . serves as the pivot about which the rotation of the head occurs . odontoid process = dens . anterior articular facet Thoracic vertebrae . spinous process is long and running posteroinferiorly . superior costal facet . inferior costal facet . transverse process has an articulating facet for the tubercle of a rib = costal facet . the body is heart-shaped Lumbar vertebrae . massive bodies . accessory process - on the posterior surface of the base of each transverse process . mammilary process - on the posterior surface of the superior articular process . costal process Sacrum solid triangular bone . base . wings (alae) . apex . dorsal surface median crest intermediate crest lateral crest posterior sacral foramina superior art. processes . -

1 the Thoracic Wall I
AAA_C01 12/13/05 10:29 Page 8 1 The thoracic wall I Thoracic outlet (inlet) First rib Clavicle Suprasternal notch Manubrium 5 Third rib 1 2 Body of sternum Intercostal 4 space Xiphisternum Scalenus anterior Brachial Cervical Costal cartilage plexus rib Costal margin 3 Subclavian 1 Costochondral joint Floating ribs artery 2 Sternocostal joint Fig.1.3 3 Interchondral joint Bilateral cervical ribs. 4 Xiphisternal joint 5 Manubriosternal joint On the right side the brachial plexus (angle of Louis) is shown arching over the rib and stretching its lowest trunk Fig.1.1 The thoracic cage. The outlet (inlet) of the thorax is outlined Transverse process with facet for rib tubercle Demifacet for head of rib Head Neck Costovertebral T5 joint T6 Facet for Tubercle vertebral body Costotransverse joint Sternocostal joint Shaft 6th Angle rib Costochondral Subcostal groove joint Fig.1.2 Fig.1.4 A typical rib Joints of the thoracic cage 8 The thorax The thoracic wall I AAA_C01 12/13/05 10:29 Page 9 The thoracic cage Costal cartilages The thoracic cage is formed by the sternum and costal cartilages These are bars of hyaline cartilage which connect the upper in front, the vertebral column behind and the ribs and intercostal seven ribs directly to the sternum and the 8th, 9th and 10th ribs spaces laterally. to the cartilage immediately above. It is separated from the abdominal cavity by the diaphragm and communicates superiorly with the root of the neck through Joints of the thoracic cage (Figs 1.1 and 1.4) the thoracic inlet (Fig. -

Foramen in Xiphoid Process of Sternum
[Sabnis et. al., Vol.7 (Iss.12): December 2019] ISSN- 2350-0530(O), ISSN- 2394-3629(P) Index Copernicus Value (ICV 2018): 86.20 DOI: https://doi.org/10.29121/granthaalayah.v7.i12.2019.317 Science FORAMEN IN XIPHOID PROCESS OF STERNUM Dr Anjali Sabnis 1, Dr. Prakash Mane 2 1 Professor and head, Department of Anatomy, MGM Medical College, Navi Mumbai 2 Tutor, Department of Anatomy, MGM Medical College, Navi Mumbai Abstract Xiphoid process is smallest and distal component of manubrium sternum which is located in epigastrium. Variations in size and shape of xiphoid process are commonly observed. During teaching sternum to 1st M.B.B.S. students of MGM Medical College, Navi Mumbai, two small foramina in two different xiphoid processes were noticed. Though presence of foramen in xiphoid process is not uncommon, their presence should not be ignored. During assessment of injuries in autopsy, post-mortem examination and radiological reporting knowledge of xiphoid process and foramina in xiphoid process will be helpful. Keywords: Foramen; Xiphoid Process; Manubrium; Sternum; Perforation. Cite This Article: Dr Anjali Sabnis, and Dr. Prakash Mane. (2019). “FORAMEN IN XIPHOID PROCESS OF STERNUM.” International Journal of Research - Granthaalayah, 7(12), 239-242. https://doi.org/10.29121/granthaalayah.v7.i12.2019.317. 1. Introduction Sternum is a flat bone which forms anterior boundary of thoracic wall. Prosternum or manubrium, mesosternum or body and metasternum or xiphoid process are the three components of sternum. The distal and smallest component is xiphoid process which is located in epigastrium. Xiphoid process is derived from the Greek word “xiphos” means straight sword1. -

Calcification of Costal Cartilage in the Adult Rib Cage
IRC-17-106 Calcification of Costal Cartilage in the Adult Rib Cage. IRCOBI conference 2017 SA. Holcombe, S. Ejima, SC. Wang Abstract Costal cartilage bridges the sternum and the ribs and plays a key role in the biomechanics of the chest. Costal cartilage is known to calcify in local regions with age, which can substantially stiffen its overall response to loading. However, the rate of accumulation of the calcified volume is not well quantified. Current computational models of the thorax assign a homogeneous soft material to cartilage segments, yet their finite element meshes are well suited to the specification of interstitial calcification zones, should applicable data become available. This study measures volumes and extents of costal cartilage calcification from 205 live subject CT scans. Significant increases in volume calcification – both in a given cartilage segment and in the lengthwise extent of those segments that experience calcification – are seen with age (p<0.0001). Age and sex accounted for 35% of all inter‐individual population variability. Specific recommendations for introducing person‐age via regional calcification to models of the costal cartilage are that (1) calcification volume within a segment should increase at the rate of 0.9 mm (for an equivalent cube edge length) per decade, and (2) should involve an increasing lengthwise extent of the cartilage segment at a rate of at least 7% per decade. Keywords age, calcification, cartilage, costal cartilage calcification, rib. I. INTRODUCTION The chest plays a major role in mitigating the forces that the body experiences during motor vehicle crash events. Costal cartilage is a key structural component of the chest, contributing to its rigidity and reinforcing the chest wall by bridging the space between the sternum and the ribs. -

Chest Wall Abnormalities and Their Clinical Significance in Childhood
Paediatric Respiratory Reviews 15 (2014) 246–255 Contents lists available at ScienceDirect Paediatric Respiratory Reviews CME article Chest Wall Abnormalities and their Clinical Significance in Childhood Anastassios C. Koumbourlis M.D. M.P.H.* Professor of Pediatrics, George Washington University, Chief, Pulmonary & Sleep Medicine, Children’s National Medical Center EDUCATIONAL AIMS 1. The reader will become familiar with the anatomy and physiology of the thorax 2. The reader will learn how the chest wall abnormalities affect the intrathoracic organs 3. The reader will learn the indications for surgical repair of chest wall abnormalities 4. The reader will become familiar with the controversies surrounding the outcomes of the VEPTR technique A R T I C L E I N F O S U M M A R Y Keywords: The thorax consists of the rib cage and the respiratory muscles. It houses and protects the various Thoracic cage intrathoracic organs such as the lungs, heart, vessels, esophagus, nerves etc. It also serves as the so-called Scoliosis ‘‘respiratory pump’’ that generates the movement of air into the lungs while it prevents their total collapse Pectus Excavatum during exhalation. In order to be performed these functions depend on the structural and functional Jeune Syndrome VEPTR integrity of the rib cage and of the respiratory muscles. Any condition (congenital or acquired) that may affect either one of these components is going to have serious implications on the function of the other. Furthermore, when these abnormalities occur early in life, they may affect the growth of the lungs themselves. The followingarticlereviewsthe physiology of the respiratory pump, providesa comprehensive list of conditions that affect the thorax and describes their effect(s) on lung growth and function. -

Asymmetric Costochondral Junction
• Initially for cardiac echo • Subsequent studies non-cardiac applications – 1973: Goldberg et al in JCUS • 30 mediastinal masses in pts. age 1-84 yrs. – 1977: Kangarloo et al in Radiology • Juxtadiaphragmatic lesions in children, value of liver window – 1980: Haller et al in AJR • 28 children - 93% success rate; evaluation of opaque hemithorax, characterizing pleural fluid, guiding drainage, integrity of diaphragm – 1984: Claus and Coppens in Ann Radiol • Value of thymus as a window for US for mediastinal masses – 1984: Miller et al in Radiology • “Water path ultrasound of chest disease in childhood” – 82 children placed in “Octoson” to expand windows – 1986: Rosenberg HK in RG • Complimentary role of US and CXR in differentiating pediatric chest abnormalities – 1989: Glasier et al in J Pediatr • Modality of choice for opaque chest, mediastinal masses, and pleural disease – 1993: Ben -Ami TE et al in RCNA • Review – 2005: Coley BD in RCNA • Review • Generic • Pediatric chest – Non invasive, inexpensive, no “contrast”, – Aerated lung realtime, Doppler, bedside/portable, • Requires parenchymal lesion to be some tissue characterization superficial • Pediatric – Older children • Sternum ossified, thymus small – No radiation – Paucity of fat – Smaller, more superficial structures • Pediatric chest – Portable – Cartilagenous sternum and ribs – Thymic window • Thoracic cage • Diaphragm • Mediastinum • Pleura & Parenchyma • Etiology of breast lumps in children can be clinically challenging • Anterior chest wall lesions can masquerade as breast -
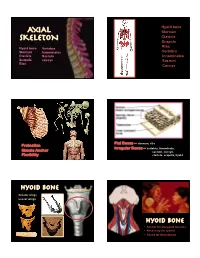
3. Axial Skeleton
Hyoid bone AXIAL Sternum SKELETON Clavicle Scapula Ribs Hyoid bone Vertebra Sternum Innominates Vertebra Clavicle Sacrum Innominates Scapula coccyx Sacrum Ribs Coccyx Flat Bones -- sternum, ribs Protection Irregular Bones -- vertebra, innominate, Muscle Anchor sacrum, coccyx, Flexibility clavicle, scapula, hyoid Hyoid Bone Greater wings Lesser wings Hyoid Bone • Anchor for pterygoid muscles • Necessary for speech • Found for Neandertals 1 Sternum Manubrium Clavicular notch Body Costal notchs Xyphoid Process sternum • Anchor for muscles • Protection for heart clavicle Sternal End Scapular End Costoclavicular ligament attachment Conoid Tubercle Acromion Coronoid Spine scapula scapula • Protection for lungs Blade • Anchor for numerous Glenoid arm and back muscles Notch 2 Vertebra RIBS Head Neck False Ribs Angle Facet • Protection for spinal cord • Anchor for numerous muscles Floating Ribs Vertebra Vertebra Intervertebral disks Cervical Vertebra Cervical Vertebra Transverse foramen 3 Atlas Axis Dens apistrophe Superior articular facets Superior articular facets Vertebral foramen Transverse foramen Transverse foramen Body Transverse process Spinous process Vertebral foramen Inferior articular facets Inferior articular facets Transverse process Spinous process Thoracic Vertebra Body Superior articular facet Vertebral foramen Transverse foramen Transverse process Spinous process Rib Facets C3 - C7 Lumbar Vertebra Thoracic Vertebra 4 L5 L1 lumbar Orientation of the Ala bones of the Thorax Promotory Vertebra Articular Surface sacrum Ribs Sternum sacrum coccyx 5 innominates innominates Acetabulum 6. -

BREASTPLATE: Sternum RIBS
Lab 4 FUNCTIONAL HUMAN ANATOMY LAB #4 THORACIC CAVITY THORACIC OSTEOLOGY: BREASTPLATE: Manubrium The joint between the Manubrium and the Sternum is called the manubrialsternal joint. This point is also a surface landmark called the sternal angle (which can be easily palpated). This is clinically Sternum important because it is located at the level of the second rib. Once the sternal angle has been palpated, the intercostal spaces may be Xiphoid process identified. RIBS: Head Neck Ribs 1-7 are true ribs (have direct connection to the sternum); 8-12 Tubercle are false ribs (no direct connection to the sternum); 11-12 are also floating ribs (have no anterior connection at all). Each rib articulates Angle with the thoracic vertebre in two places. The head of the rib articulates with the body of a vertebre and the tubercle of the rib articulates with Costal groove the tranverse process of a vertebre. There are costal facets present at these articulation points. Sternal end Costal chondrial joint THORACIC MUSCULATURE: Pectoralis major Medial attachment: consists of three heads (clavicular, sternal and Proximal end of clavical, body of sternum and the middle few ribs abdominal); only identify for now, we will discuss the function of this muscle in the Lateral attachment: upper extremity lab Lateral lip of interturbicular (bicipital) groove Function: Flexion of the arm at shoulder (clavicular head); horizontal adduction (sternal head); flexion of arm at shoulder from a hyperextended position (abdominal head); horizontal adduction and internal -

Sternal Fracture
Sternal fracture What is a sternal fracture? The sternum or breastbone is the bone in the front and center of the chest. Your ribs and clavicle (collar bone) attach to your sternum. A sternal fracture is a break in this bone. The most common cause of a sternal fracture is trauma. It can occur with direct blow to front of your chest, fall or motor vehicle accidents where your chest hits the steering wheel or seat belt. Common symptoms include: Chest pain at time and site of injury Pain with breathing, coughing, or sneezing Shortness of breath Bruising and tenderness of front of chest. Diagnosis: The fracture may have been found with an x-ray or CT scan. An EKG or ultrasound may be performed to look at your heart, major blood vessels, and lungs to make sure there is no damage to these organs. Treatment: Most sternal fractures will heal in several weeks and do not require surgery. Treatment will often be pain control and avoiding activities that may reinjure your sternum. It is important to continue to take deep breaths and cough to prevent pneumonia. It is important you do not smoke as this will slow bone healing. Continue to take your pain medications as prescribed. These are some precautions to help you while your sternum heals: - Brace your chest when coughing, sneezing, or turning. You will need to hold a pillow or cross your arms over your chest (this is called splinting). This is most important to prevent pneumonia. - Do not lift push or pull anything over 10 pounds. -

Bone Diagram.Pub
Bone Diagram Forehead The common name of (Frontal bone) each bone is listed first, Nose bones with the scientific name (Nasals) given in parenthesis. Cheek bone (Zygoma) Upper jaw (Maxilla) Lower jaw (Mandible) Collar bone (Clavicle) Breast bone (Sternum) Upper arm bone (Humerus) Lower arm bone (Ulna) Lower arm bone (Radius) Thigh bone (Femur) Kneecap (Patella) Shin bone (Tibia) Calf bone (Fibula) Ankle bones (Tarsals) Foot bones (Metatarsals) Toe bones (Phalanges) Skull Side of skull (Cranium) (Parietal bone) Back of skull Did you know? (Occipital bone) When you are a baby you Temple have more than 300 (Temporal bone) bones. By the time you are an adult you only Backbone Neck vertebrae (7) have 206 bones, because (Spine) (Cervical vertebrae) some of your bones join together as you grow! Shoulder blade (Scapula) Chest vertebrae (12) (Thoracic vertebrae) Ribs (12 pairs) Lower back vertebrae (5) (Lumbar vertebrae) Fused vertebrae (5) (Sacrum) Pelvic bones (Ilium) (Pubis) (Ischium) Wrist bones (Carpals) Hand bones (Metacarpals) Finger bones Bones are important! (Phalanges) They hold up your body, and along with your muscles, keep you moving. Without your bones, you’d just be one big blob! To be able to grow, strong bones needs lots of calcium and weight-bearing physical activity. Heel bone (Calcaneus) University of Washington PKU Clinic CHDD - Box 357920, Seattle, WA 98195 (206) 685-3015, Toll Free in Washington State 877-685-3015 http://depts.washington.edu/pku .