Pollinator-Driven Divergence Among Populations of a Self-Fertilizing Lily, Hesperantha Coccinea (Iridaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2Nd International Congress of Alpine and Arctic Botanical Gardens
Proceedings of the 2nd International Congress of Alpine and Arctic Botanical Gardens München 22-25 April 2009 CONTENTS • Introduction........................................................ 5 • Christine Freitag (Freising, Germany) Educative tools to connect an alpine garden Diversification of Collections to the surrounding vegetation......................... 35 • Katie Price (Kew, United Kingdom) • Jenny Wainwright-Klein (München, Germany) Kew’s Alpine House - what’s the point?......... 39 Experiences with the introduction of southern hemisphere alpines.............................................. 6 Research and Conservation Activities • Richard Hurstel, Pascal Salze, Christophe Per- rier, Rolland Douzet & Serge Aubert (Grenoble, • Gunter Karste (Wernigerode, Germany) France) Investigation on renaturation of the subalpine Experiences with the introduction of southern meadow vegetation on top of Brocken mountain hemisphere alpines: Southern Andes and Pata- ............................................................................. 44 gonia...................................................................... 9 • Andreas Gröger & Annette Menzel (München & • Anne Humburg (Seligenstadt, Germany) Freising, Germany) Betty Ford Alpine Gardens: the many faces of Detection of climate change impacts in alpine North America’s highest botanical garden...... 13 and arctic botanic gardens: a long-term pheno- logy observation program............................... 47 Horticultural Practices • George Nakhutsrishvili, Sh. Sikharulidze (Tbilisi, Georgia) -

Cambo Plant Sales - June 2020
CAMBO PLANT SALES - JUNE 2020 In addition to items on this list, we have the gift shop products available too. Please add a note to your order and Charlotte will call you from the giftshop to talk you through some of our lovely local handmade gifts and products. (eg. A birthday card for a female and a small gift for around £10) Category (from KT Item Size Description Price Availability original spreadsheet) 1 Garden product Bee Bomb Bee bomb flower seeds contain 1000s of seeds 7.99 Now from 18 native wildflower species, mixed with protective clay and top soil. 1 pack provides coverage for roughly 21 sq ft / 2m². 2 Garden product Buds Garden Gloves - Childrens XXS (4-6 A durable and easy to wear kids glove that will 3.99 Now yrs) keep little hands safe whilst gardening outside. 3 Garden product Buds Garden Gloves - Childrens XS (7-9 yrs) A durable and easy to wear kids glove that will 3.99 Now keep little hands safe whilst gardening outside. 4 Garden product Darlac Bypass Pruner (Expert Little pressure is required in pruning of this nature 25.99 Now Bypass Pruner) so the pruners are lightweight. SK5 high carbon steel. 5 Garden product Darlac garden trowel For general gardening and potting. Eco friendly 9.95 Now bamboo handle & stainless steel head 6 Garden product Darlac lightweight sheers Extra long handles for greater reach, replaceable 17.99 Now carbon steel blades, Razor sharp 8" carbon steel blades, For grass, hedge trimming and topiary 7 Garden product Melcourt peat-free Sylvagrow 50L Used in the gardens at Cambo 11.50 Now compost Category (from KT Item Size Description Price Availability original spreadsheet) 8 Garden product Nutscene twine spool - Nutscene Twist Garden Twine. -

PERENNIAL PLANTS Plant Name Common Name Height Colour Bl Time Special Conditions Country S
PERENNIAL PLANTS Plant Name Common Name Height Colour Bl Time Special Conditions Country S. Europe, NW Acanthus mollis Bear's Breeches to 5' (1.5m) white fls. with purple shaded bracts l summer z7 sun/pt.shade,well drained, moist good soil Africa Acanthus spinosus Bear's Breeches to 5' (150cm) white flowers with purple bracts lsp-msum z5 sun/pt.shade, good soil, tolerates dry heat Italy to W Turkey Aconitum Monkshood large dark blue flowers l summer z5 sun/part shade, cool moist fertile soil Aconitum Monkshood dark blue flowers l summer z5 sun/part shade, cool moist fertile soil Monkshood (all parts are Aconitum carmichaelii to 6' (190cm) violet or blue flowers l sp to fall z3 sun/pt.shade, cool, moist, fertile soil Russia poisonous) Monkshood (all parts are Aconitum carmichaelii 'Barker's Variety' to 6' (190cm) deep violet flowers early fall z3 sun/pt.shade, cool, moist, fertile soil poisonous) Aconitum 'Ivorine' (syn.A.septentrionale Monkshpood (all parts are to 36" (90cm) ivory flowers l spring z5 sun/pt.shade, cool, moist, fertile soil garden origin 'Ivorine') poisonous) Aconitum lycoctonum ssp.vulparia Monkshood (all parts are to 5' (1.5m) pale yellow flowered form sum/e fall z4 sun/pt.shade, cool, moist, fertile soil Europe (A.orientale of gardens) poisonous) Acorus gramineus 'Variegatus' Variegated Japanese Rush to 10" (25 cm) creamy white and green striped leaves summer z5 full sun, wet or very moist soil E Asia z4 shade/pt.sh.moist mod-fertile soil.Survives under Actaea erythrocarpa (syn. A.spicata var. rubra) 24" (60cm) racemes of white flowers,red berries late spring Euro. -

Plants at MCBG
Mendocino Coast Botanical Gardens All recorded plants as of 10/1/2016 Scientific Name Common Name Family Abelia x grandiflora 'Confetti' VARIEGATED ABELIA CAPRIFOLIACEAE Abelia x grandiflora 'Francis Mason' GLOSSY ABELIA CAPRIFOLIACEAE Abies delavayi var. forrestii SILVER FIR PINACEAE Abies durangensis DURANGO FIR PINACEAE Abies fargesii Farges' fir PINACEAE Abies forrestii var. smithii Forrest fir PINACEAE Abies grandis GRAND FIR PINACEAE Abies koreana KOREAN FIR PINACEAE Abies koreana 'Blauer Eskimo' KOREAN FIR PINACEAE Abies lasiocarpa 'Glacier' PINACEAE Abies nebrodensis SILICIAN FIR PINACEAE Abies pinsapo var. marocana MOROCCAN FIR PINACEAE Abies recurvata var. ernestii CHIEN-LU FIR PINACEAE Abies vejarii VEJAR FIR PINACEAE Abutilon 'Fon Vai' FLOWERING MAPLE MALVACEAE Abutilon 'Kirsten's Pink' FLOWERING MAPLE MALVACEAE Abutilon megapotamicum TRAILING ABUTILON MALVACEAE Abutilon x hybridum 'Peach' CHINESE LANTERN MALVACEAE Acacia craspedocarpa LEATHER LEAF ACACIA FABACEAE Acacia cultriformis KNIFE-LEAF WATTLE FABACEAE Acacia farnesiana SWEET ACACIA FABACEAE Acacia pravissima OVEN'S WATTLE FABACEAE Acaena inermis 'Rubra' NEW ZEALAND BUR ROSACEAE Acca sellowiana PINEAPPLE GUAVA MYRTACEAE Acer capillipes ACERACEAE Acer circinatum VINE MAPLE ACERACEAE Acer griseum PAPERBARK MAPLE ACERACEAE Acer macrophyllum ACERACEAE Acer negundo var. violaceum ACERACEAE Acer palmatum JAPANESE MAPLE ACERACEAE Acer palmatum 'Garnet' JAPANESE MAPLE ACERACEAE Acer palmatum 'Holland Special' JAPANESE MAPLE ACERACEAE Acer palmatum 'Inaba Shidare' CUTLEAF JAPANESE -

The Interplay Between Inflorescence Development and Function As the Crucible of Architectural Diversity
Annals of Botany Page 1 of 17 doi:10.1093/aob/mcs252, available online at www.aob.oxfordjournals.org REVIEW: PART OF A SPECIAL ISSUE ON INFLORESCENCES The interplay between inflorescence development and function as the crucible of architectural diversity Lawrence D. Harder1,* and Przemyslaw Prusinkiewicz2 1Department of Biological Sciences, University of Calgary, Calgary, Alberta, Canada T2N 1N4 and 2Department of Computer Science, University of Calgary, Calgary, Alberta, Canada T2N 1N4 * For correspondence. Email [email protected] Received: 27 July 2012 Returned for revision: 13 September 2012 Accepted: 17 October 2012 † Background Most angiosperms present flowers in inflorescences, which play roles in reproduction, primarily Downloaded from related to pollination, beyond those served by individual flowers alone. An inflorescence’s overall reproductive contribution depends primarily on the three-dimensional arrangement of the floral canopy and its dynamics during its flowering period. These features depend in turn on characteristics of the underlying branching structure (scaffold) that supports and supplies water and nutrients to the floral canopy. This scaffold is produced by devel- opmental algorithms that are genetically specified and hormonally mediated. Thus, the extensive inflorescence diversity evident among angiosperms evolves through changes in the developmental programmes that specify http://aob.oxfordjournals.org/ scaffold characteristics, which in turn modify canopy features that promote reproductive performance in a par- ticular pollination and mating environment. Nevertheless, developmental and ecological aspects of inflorescences have typically been studied independently, limiting comprehensive understanding of the relations between inflor- escence form, reproductive function, and evolution. † Scope This review fosters an integrated perspective on inflorescences by summarizing aspects of their devel- opment and pollination function that enable and guide inflorescence evolution and diversification. -

TURF REPLACEMENT PROGRAM MMWD LYL Approved Plant List
LANDSCAPE YOUR LAWN (LYL) TURF REPLACEMENT PROGRAM MMWD LYL Approved Plant List Attached is the current MMWD list of approved plants for the The values are obtained by determining the area of a circle using Landscape Your Lawn (LYL) Program. the plant spread or width as the diameter. To find the area of a circle, square the diameter and multiply by .7854. Squaring the This list is taken from the Water Use Classification of Landscape diameter means multiplying the diameter by itself. For example, a Species (WUCOLS IV) – a widely accepted and commonly used plant with a 5 foot spread would be calculated as follows: source of information on landscape plant water needs. Plants that .7854 x 5 ft diameter x 5 ft diameter = 20 sq ft (values are rounded are listed in WUCOLS IV as “low” or “very low” water use for the Bay to the nearest whole number). Area have been included on this list. However, plants that are considered invasive and are found on the MMWD Invasive Plant List For values not provided, please refer to reputable gardening books are not included in this list and will not be allowed for the LYL or nurseries in order to determine the diameter of the plant at program. maturity, or conduct an internet search using the botanical name and “mature size”. Any plants used in turf conversion that are not on this plant list will not count toward the 50 percent plant coverage requirement nor CA Natives will they be eligible for a rebate under LYL Option 1. Native plants are perfectly suited to our climate, soil, and animals. -

WUCOLS List S Abelia Chinensis Chinese Abelia M ? ? M / / Copyright © UC Regents, Davis Campus
Ba Bu G Gc P Pm S Su T V N Botanical Name Common Name 1 2 3 4 5 6 Symbol Vegetation Used in Type WUCOLS List S Abelia chinensis Chinese abelia M ? ? M / / Copyright © UC Regents, Davis campus. All rights reserved. bamboo Ba S Abelia floribunda Mexican abelia M ? M M / / S Abelia mosanensis 'Fragrant Abelia' fragrant abelia ? ? ? ? ? ? bulb Bu S Abelia parvifolia (A. longituba) Schuman abelia ? ? ? M ? ? grass G groundcover GC Gc S Abelia x grandiflora and cvs. glossy abelia M M M M M / perennial* P S Abeliophyllum distichum forsythia M M ? ? ? ? palm and cycad Pm S Abelmoschus manihot (Hibiscus manihot) sunset muskmallow ? ? ? L ? ? T Abies pinsapo Spanish fir L L L / / / shrub S succulent Su T N Abies spp. (CA native and non-native) fir M M M M / / P N Abronia latifolia yellow sand verbena VL VL VL / ? ? tree T P N Abronia maritima sand verbena VL VL VL / ? ? vine V California N native S N Abutilon palmeri Indian mallow L L L L M M S Abutilon pictum thompsonii variegated Chinese lantern M H M M ? ? Sunset WUCOLS CIMIS ET Representative Number climate 0 Region zones** Cities zones* S Abutilon vitifolium flowering maple M M M / ? ? Healdsburg, Napa, North- San Jose, Salinas, Central 14, 15, 16, 17 1, 2, 3, 4, 6, 8 San Francisco, Coastal San Luis Obispo S Abutilon x hybridum & cvs. flowering maple M H M M / / 1 Auburn, Central Bakersfield, Chico, 8, 9, 14 12, 14, 15, 16 Valley Fresno, Modesto, Sacramento S T Acacia abyssinica Abyssinian acacia / ? / ? / L 2 Irvine, Los South Angeles, Santa 22, 23, 24 1, 2, 4, 6 Coastal Barbara, Ventura, -

Sheet1 Haberlea Ferdinandi-Coburgii 'Connie Davidson' £5.80 Needing
Sheet1 Haberlea ferdinandi-coburgii 'Connie £5.80 Needing cool, moist shade, this forms more or less evergreen Davidson' rosettes of thick textured, deeply veined leaves. Clusters of narrowly tubular, violet-blue flowers with a white marked throat, during late spring. A challenging plant for East Anglia. (4-5) 10cm. Hedychium aurantiacum £5.50 One of the hardier species with erect stems topped by spikes of rich golden-yellow flowers with deeper filaments in late autumn & early winter. Only hardy in sheltered, well drained mild spots in sun. (9-11) 120cm. Hedychium coronarium £5.20 Upright perennial, with pointed dark-green leaves, downy beneath. Veryt fragrant butterfly like white flowers with a yellow base are borne in cylindrical spikes in mid to late summer. hardy only in a very sheltered well drained spot in sun. (8-9) 300cm Hedychium densiflorum £5.20 Upright clump former, with lance-shaped glossy green leaves. Dense cylindrical spikes of fragrant pale-orange flowers with cream filaments. One of the hardier species for a very sheltered well drained spot in the south. (8-9) 400cm. Hedychium densiflorum 'Stephen' £5.80 Densely packed, compact cylindrical spikes of pale orange-yellow flowers, with striking orange anthers. Well scented. Pointed leaves on upright stems. Flowers during late summer in a very sheltered, well drained spot in sun. Marginally hardy. (8-9) 180cm. Hedychium ellipticum £5.20 Dense spikes of pale yellow-white flowers, with two-lobed lower lips & unusual purple filaments. Flowers late summer & autumn. Upright stems, with broad dark-green leaves. Marginally hardy in a sheltered well drained spot in sun. -
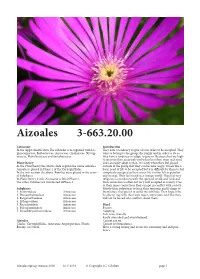
Aizoales 3-663.20.00
Aizoales 3-663.20.00 Taxonomy Introduction In the Apg2 classifcation Te suborder is recognised with Lo- Tey have a tendency to give a lot in order to be accepted. Tey phiocarpaceae, Barbeuiaceae, Aizoaceae, Gisekiaceae, Nyctag- want to belong to the group, the family and in order to do so inaceae, Phytolaccaceae and Sarcobataceae. they have a tendency to adapt, to give in. Because they are high- ly sensitive they accurately feel what the others want and need Plant theory and can easily adapt to that. It is only when they feel placed In the Plant theory the above clade is given the name Aizoales. outside of the group that they can become angry. It feels like a Aizoales is placed in Phase 2 of the Caryophyllidae. basic need of life to be accepted but it is difcult for them to feel In the frst version the above Families were placed in the sever- completely accepted as their inner life is ofen felt as peculiar al Subphases. and strange. Tey feel weird in a strange world. Tey feel very In Plant theory 2 only Aizoaceae is lef inPhase 2. religious, a connection with the spiritual world and God and Te other Families are transferred toPhase 3. that connection is ofen not very well accepted in society. Due to their inner convictions they can get in confict with society. Subphases Mostly their solution is to keep their opinions and feelings to 1. Sesuvioideae Aizoaceae themselves; they prefer to avoid the conficts. Tey hope to be 2. Drosanthemoideae Aizoaceae be able to stay with their own inner convictions and that they 3. -

HORTAX Cultivated Plant Taxonomy
Cultivated Plant TaxonomyNS EW Issue 2 ■ June 2014 Culture and taxonomy: new tools for a new understanding James Armitage EDITOR As Chairman of Hortax, one of the things I am enthusiastic to impress on people is the cultural importance of cultivated plants. When they have undergone a process of selection or breeding, plants become examples of living history, forever connected to customs, fashions, events, places and people. In time they can even take on a symbolic significance in folklore, myth and legend. Above, clockwise. Rosa gallica ‘Versicolor’, seen through first-century Pompeian, I was particularly struck by these ideas nineteenth-century Belgian, and twentieth-century English eyes. when visiting China last July to attend the Sixth International Symposium on her thoughts on the Symposium and PHOTOS ( the Taxonomy of Cultivated Plants in analyses the development of these Hortax on CLOCKWISE Beijing. As a European, I had not before conferences over time. In the second, had the opportunity to meet so many Camellia expert Twitter Jennifer Trehane ). Asian researchers, and I came away discusses how taxonomic information Hortax is now social STEFANO with the strong impression that the can be lost in translation when plants networking! The cultural relevance of the plants they are moved around the globe. Hortax Twitter feed BOLOGNINI were investigating was an important offers links to ; ; factor in their work. I hope their Also in this edition, Mike Grant news and RHS , , message that taxonomic study is a makes the case for The Plantsman observations LINDLEY tool that can be used to examine the as a place to publish horticultural from the LIBRARY heritage that plants represent will come taxonomy and Penny Maplestone world of ; ; to be understood more widely. -

Scientific Name Common Name Family Score Acorus Calamus Sweet Flag
Scientific name Common name Family Score Acorus calamus Sweet flag Acoraceae 48 Acorus gramineus Grass-leaf sweet flag Acoraceae 20 Aldrovanda vesiculosa Waterwheel plant Droseraceae 27 Alisma plantago-aquatica Water plantain Alismataceae 37 Alternanthera philoxeroides Alligator weed Amaranthaceae 75 Aponogeton distachyos Cape pondweed Aponogetonaceae 53 Aponogeton natans Floating lace plant Aponogetonaceae 15 Azolla pinnata Mosquito fern Azollaceae 67 Bolbitis heteroclita Asian water fern Lomariopsidaceae 23 Butomus umbellatus Flowering rush Butomaceae 62 Cabomba caroliniana* Fanwort Cabombaceae 67 Callitriche stagnalis European water starwort Callitrichaceae 42 Canna × generalis Common garden canna Cannaceae 26 Cardamine lyrata Chinese ivy Brassicaceae 21 Crassula helmsii Australian stonecrop Crassulaceae 70 Cyperus difformis Small flower umbrella plant Cyperaceae 45 Cyperus involucratus Umbrella sedge Cyperaceae 35 Cyperus longus Sweet cyperus Cyperaceae 19 Cyperus serotinus Tidal marsh flat sedge Cyperaceae 27 Egeria densa Brazilian elodea Hydrocharitaceae 71 Eichhornia crassipes Water hyacinth Pontederiaceae 81 Elatine macropoda Southern waterwort Elatinaceae 14 Eriophorum latifolium Grey cotton grass Cyperaceae 11 Euryale ferox Gorgon Nymphaeaceae 19 Glyceria fluitans Floating manna grass Poaceae 45 Glyceria maxima Reed sweet grass Poaceae 71 Gratiola officinalis Gratiola Plantaginaceae 28 Scientific name Common name Family Score Gratiola peruviana Austral brooklime Plantaginaceae 23 Hesperantha coccinea River lily Iridaceae 28 -
Manzanita House Plant List for Mark Kuestner's Garden [Plants on the Property When We Moved In, November 2015]
Manzanita House Plant List For Mark Kuestner’s Garden [Plants on the property when we moved in, November 2015]: Azaleas Bergenia Butterfly Bush Camellias Cherry, Flowering Japanese (weeping form) Fuchsias (tall, hardy) Grasses, Ornamental (various) Hemlock Trees Hesperantha coccinea (formerly: Schizostylis coccinea) Hydrangea (mop-head, purple-blue flowering) Huckleberry, evergreen (native: Vaccinium ovatum) Ivy, English (variegated white leaf) Lychnis Coronaria (Rose campion) Magnolia, purple flowering, March-April (deciduous) Maple Trees (Silver and Japanese) Miscanthus Sinensis Gracillimus (Chinese Maidenhair Grass) Morella Californica (aka Myrica Californica formerly) Pacific Wax Myrtle (see Morella) Pine Trees Rhododendrons Rubus calycinoides (Creeping Bramble) Salal Viburnums (various) Plants added after November 2015: Abies koreana ‘Kohouts Ice Breaker’, Korean fir Abutilon ‘Red Tiger’ (Flowering Maple) Abutilon ‘Souvenir de Bonn’ Abutilon hybrid ‘Canary Bird’ Abutilon hybrid ‘Megapotamicum’ Red Abutilon hybrid ‘Victorian Lady’ Abutilon 'Talini's Pink' "Flowering Maple" Acacia cognata ‘Mini Cog’ (Cousin Itt) Acacia Pravissima Acacia Stenophylla Shoe String Acacia Acaena saccaticulpula ‘Blue’ Acanthus ‘Whitewater’ (Variegated bear’s breeches) Acidanthera Murielae (aka Gladiolus) Aeschynanthus garrettii Agapetes serpens Agastache ‘Kudos Ambrosia’ Agastache aurantiaca ‘Coronado’ Agastache Mexicana ‘Sangria’ Agastache rugosa ‘Heronswood Mist’ Agave parryi var. truncate ‘Artichoke Agave’ Agave vilmoriniana ‘Stained Glass’ Agrostemma