Mineral Sciences Investigations 1976-1977
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RESALE Numberor Stating You Are a Retailor In
TucsonAuction08.html 9th Annual Tucson Meteorite Auction ----------------------------- Tucson Meteorite Auction 2008 Saturday, February 9th, 2008 Bidding starts 7:30PM Sharp Viewing & Socializing begins 5:30PM Food and Drink available http://www.michaelbloodmeteorites.com/TucsonAuction08.html (1 z 36) [2008-05-28 18:09:44] TucsonAuction08.html (Please drink only with a designated driver) ----------------------- While in Tucson I will have a cell phone: (619) 204-4138 (Feb2-Feb10) NEW LOCATION VFW Hall (Post # 549) 1884 So. Craycroft, Tucson, AZ 85711 (see directions below) NOTE: Click HERE for printer friendly copy of this catalog (Click on any photo to see a greatly enlarged image) 1 AH 1 Claxton L6, GeorgiaDecember 10 th , 1984 - Hit A Mailbox! .992g Rim Crusted Part Slice (21mm X 20mm X 2mm) No Minimum 2 AH 2 Dhofar 908 Lunar Meteorite - Rosetta - 1.242g Full Slice (24mm X 16mm X 2mm) No Minaimum - (est: $2.5K min) 3 AH 3 NWA 2999 Angrite Famous Paper "The Case For Samples From Mercury" 3.216g FC End Piece (18mm X 15mm X 7mm) No Minimum http://www.michaelbloodmeteorites.com/TucsonAuction08.html (2 z 36) [2008-05-28 18:09:44] TucsonAuction08.html 4 AH 4 NWA 4473 Polymict Diogenite 13g Full Slice(70mm X 13mm X ~2.5mm) No Minimum 5 AH 5 NWA 4880 (Shergottite) .540g 70% F Crusted Whole Stone (11mm X 9mm X 5mm) No Minimum 6 AH 6 NWA 4880 (Shergottite) 32.3g 92% FC Oriented Main Mass (35mm X 32mm X 32mm) Minimum Bid: $12,900.00 (Less Than $400/g) 7 AH 7 Oued el Hadjar (LL6) Fall March 1986 - "The Wedding Stone" 6.322 g (41mm X 30m X 3mm) The stone was broken into many pieces, then sacrificed on an alter during a wedding ceremony. -

Zinc and Copper Isotopic Fractionation During Planetary Differentiation Heng Chen Washington University in St
Washington University in St. Louis Washington University Open Scholarship Arts & Sciences Electronic Theses and Dissertations Arts & Sciences Winter 12-15-2014 Zinc and Copper Isotopic Fractionation during Planetary Differentiation Heng Chen Washington University in St. Louis Follow this and additional works at: https://openscholarship.wustl.edu/art_sci_etds Part of the Earth Sciences Commons Recommended Citation Chen, Heng, "Zinc and Copper Isotopic Fractionation during Planetary Differentiation" (2014). Arts & Sciences Electronic Theses and Dissertations. 360. https://openscholarship.wustl.edu/art_sci_etds/360 This Dissertation is brought to you for free and open access by the Arts & Sciences at Washington University Open Scholarship. It has been accepted for inclusion in Arts & Sciences Electronic Theses and Dissertations by an authorized administrator of Washington University Open Scholarship. For more information, please contact [email protected]. WASHINGTON UNIVERSITY IN ST. LOUIS Department of Earth and Planetary Sciences Dissertation Examination Committee: Bradley L. Jolliff, Chair Jeffrey G. Catalano Bruce Fegley, Jr. Michael J. Krawczynski Frédéric Moynier Zinc and Copper Isotopic Fractionation during Planetary Differentiation by Heng Chen A dissertation presented to the Graduate School of Arts & Sciences of Washington University in partial fulfillment of the requirements for the degree of Doctor of Philosophy December 2014 St. Louis, Missouri Copyright © 2014, Heng Chen All rights reserved. Table of Contents LIST OF FIGURES -

NEW HIGH-PRECISION POTASSIUM ISOTOPES of TEKTITES. Y. Jiang1,2, H
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 1311.pdf NEW HIGH-PRECISION POTASSIUM ISOTOPES OF TEKTITES. Y. Jiang1,2, H. Chen2, B. Fegley, Jr.2, K. Lodders2, W. Hsu3, S. B. Jacobsen4, K. Wang (王昆)2. 1CAS Key Laboratory of Planetary Sciences, Purple Moun- tain Observatory, Chinese Academy of Sciences ([email protected]), 2Dept. Earth & Planetary Sciences and McDonnell Center for the Space Sciences, Washington University in St. Louis ([email protected]), 3Space Sci- ence Institute, Macau University of Science and Technology, 4Dept. Earth & Planetary Sciences, Harvard University. Introduction: Tektites are natural glassy objects crater of Hawaii (BHVO-1), are also analyzed. Hainan formed from the melting and rapid cooling of terrestri- Tektite is a typical splash-form tektite from Hainan al rocks during the high-energy impacts of meteorites, Island, China and of a dumbbell shape. We analyzed K comets, or asteroids upon the surface of the Earth [1]. isotopes of 15 chips along the longitudinal axis of its Chemical and isotopic compositions indicate that pre- profile, on a Neptune Plus MC-ICP-MS at Washington cursor components of tektites are the upper terrestrial University in St. Louis [14]. Except for Hainan tektite, continental crust, rather than extraterrestrial rocks. all other samples were only analyzed in bulk, with a Tektites are characterized by the depletion of volatile GV Instruments IsoProbe P MC-ICP-MS at Harvard elements and water, and heavy Cd, Sn, Zn and Cu iso- University following the description in [13]. K isotope topic compositions [2-5]. Since volatilities of elements compositions are reported using the per mil (‰) nota- 41 41 39 41 39 are defined by their 50% condensation temperature (Tc) tion, where δ K = ([( K/ K)sample/( K/ K)standard – of the solar nebula gas at 10-4 bars [6], there is no rea- 1]×1000). -

The 3D.Y Knom Example Is /1"<
t Sixth International Congress on Glass - Washington, D. C., 1962. Fossil Glasses Produced by Inpact of Meteorites, Asteroids 2nd Possibly Comets with the Planet Xarth* A. J. Zzhen :&uon Insticute, Pittsburgh, ?ennsylvania (U. S. A. ) Sunnary / (to be trmslzted izto Frerzh and German) - i in recent thes one of the nost intriging aysteries of geGlogy has ceen the occurrence of aerodgnasicdly-shaped glasses on five continents of the earth. Tnese glasses mder discussion are obviously not of f-d- guritic origin. 3ecent research indicates that these glasses laom as tektites are the result of meteorite, esteroid, or sossibly comet hpact. Lqact glass?s, io generzl, differ Tram volcanic glasses in that they are lo;;.tr in ?,iater zontent, have laver gallium and germiun ccntents, and are rot necessarily ia mgnaticalljr unstable continental areas. These hpac- tites may be divided as follovs: (1)Glasses found in or near terrestrial neteorite craters. These glasses usually contain numerous s-,'nerules 02 nickel-iron, coesite, chunlks of partially melted meteoritic inatter and even stishovite. Shattered or fractured melted mi-nerals such as quarts are comxonly gresent. Aerodpaaic-shaping nay or nay not be present in this t-ne. &m?les are Canyon Diablo and Wabar Crster glasses. (2) Impzct- glasses zssociated with craters uitn no evidence of meteoritic mterial i? the @.ass or surrounding the explosisn site. The 3d.y knom example is /1"< Tnis vork vas supported by Xatiocal A-eroEautizs and Space P.dministretioq,$ 3esezrch Grant NsG-37-6O Supplement 1-62. Page 2 glass associated with AoueUoul Crater in the Western Sahara Desert. -

1962-11 Millman
MINUTES SPECIAL COUNCIL MEETING OF THE METEORITICAL SOCIETY held at Los Angeles, Calif., Sat., 17 Nov. 1962 PRESENT: President Peter M. Millman, Past-President John A. Russell, Editor Dorrit Hoffleit, Councilor C. H. Cleminshaw, and Secretary Gerald L. Rowland. Since Peter Millman and Dorrit Hoffleit were both in Los Angeles at the same time, and since it was possible to get a quorum of the Council together, President Millman called a Special meeting of the Council for Sat., 17 Nov. 1962. The last Council minutes (of 4 Sept., 1962) were read in order that those present might know the business that was transacted at Socorro, New Mexico. After some discussion, it was moved, seconded, and approved that arrangements be made for the transfer of a suitable number (approximately 40) of the 4 issues of Vol. I of Meteoritics from Springer Transfer Co. in Albuquerque, N. M., to John Russell at U.S.C. The Council approved the expenditure of whatever funds are necessary for this operation. John Russell reported that the Leonard Memorial Medal, which was displayed at the Socorro meeting, was unsatisfactory and that it is being re-done by the Southern California Trophy Co. The Council was reminded that the fund established for the Leonard Memorial Medal is still open for contributions. The cost of engraving and striking each individual medal to be presented will call for a nominal expenditure from time to time. The Council next suggested the following chairmen (subject to the individual's acceptance) for the standing committees of the Society: Eugene Shoemaker (Membership and Fellowship); Oscar Monnig (Finance and Endowment); Editor Dorrit Hoffleit (Publications). -
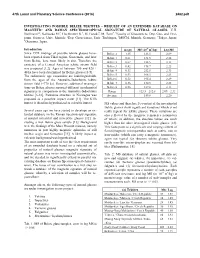
Investigating Possible Belize Tektites – Request of an Extended Database on Magnetic and Raman Spectroscopical Signature of Natural Glasses
47th Lunar and Planetary Science Conference (2016) 2482.pdf INVESTIGATING POSSIBLE BELIZE TEKTITES – REQUEST OF AN EXTENDED DATABASE ON MAGNETIC AND RAMAN SPECTROSCOPICAL SIGNATURE OF NATURAL GLASSES. V.H. Hoffmann1,2, Kaliwoda M.3, Hochleitner R.3, M. Funaki4, M. Torii5. 1Faculty of Geosciences, Dep. Geo- and Envi- ronm. Sciences, Univ. Munich; 2Dep. Geosciences, Univ. Tuebingen, 3MSCM, Munich, Germany; 4Tokyo, Japan; 5Okayama, Japan. Introduction m (gr) MS (10-9 m3/kg) Log MS Since 1992 findings of possible tektite glasses have- Belize A 1.35 123.5 2.09 been reported from Tikal region, Guatemala, and later Belize 1 0.61 131.5 2.12 from Belize, here most likely in situ. Therefore the Belize 2 0.67 128.5 2.11 existence of a Central American tektite strewn field Belize 3 0.42 178.7 2.25 was proposed [1,2]. Ages of between 780 and 820 ± Belize 4 0.33 212.8 2.33 40 ka have been determined for Belize glasses [3-5]. The radiometric age constraints are indistinguishable Belize 5 0.53 168.3 2.23 from the ages of the Australite-Indochinite tektite Belize 6 0.52 195.4 2.29 strewn field (~770 ka). However, additional investiga- Belize 7 0.36 170.5 2.23 tions on Belize glasses reported different geochemical Belize 8 0.56 129.0 2.11 signatures in comparison to the Australite-Indochinite Range 123.5 – 212.8 2.09 – 2.33 tektites [6-10]. Pantasma structure in Nicaragua was Average 159.8 2.20 proposed as a possible impact crater [11]. -

The Oscar E. Monnig Meteorite Collection and Museum. Rg
41st Lunar and Planetary Science Conference (2010) 1162.pdf THE OSCAR E. MONNIG METEORITE COLLECTION AND MUSEUM. R. G. Mayne1 and T. Moss1 De- partment of Geology, TCU Box 298830, Texas Christian University, Fort Worth, TX 76109 ([email protected]) The Man Behind the Meteorites [1]: Oscar not knowing the full extent of what was to come. In Monnig was born in Fort Worth, Texas in 1902, and he the years that followed a number of meteorites were lived there till his death in 1999. He was a business- transferred to TCU, but it was not until his death in man and he started work for his family’s dry goods 1999 that the full generosity of Oscar’s offer came to company after earning his law degree from the Univer- light. TCU not only inherited all of Oscar’s meteorite sity of Texas in 1925 and was the CEO of the company collection, but also a large amount of his estate for when it was sold in 1982. However, Oscar’s passion “education, care, and maintenance of the meteorite was for meteorites, he was an avid collector. He even collection.” gave self-printed brochures to his traveling salesmen to The Monnig Meteorite Collection at TCU: The distribute that described how to identify meteorites and collection donated by Oscar Monnig was one of the offered to purchase any that were found. finest private collections of its day, and it is now one In 1960 Oscar Monnig introduced himself to a new of the finest University-based collections in the world, hire in the TCU Geology department, Dr. -

O Lunar and Planetary Institute Provided by the NASA Astrophysics Data System the BELLS CARBONACEOUS CHONDRITE
BELLS-A CARBONACEOUS CHONDRITE RELATED TO C1 AND C2 CHONDRITES; Andrew M. ~avisland Edward 01sen2. l~amesFranck Institute, University of Chicago, 5640 South Ellis Avenue, Chicago, IL 60637; 2~epartmentof Geology, Field Museum of Natural History, Chicago, IL 60605. The Bells, Texas meteorite fell on September 9, 1961. It was classified as a Type I1 carbonaceous chondrite by Mason [I]. Bulk samples of Bells have been found to contain 13 to 16 wt% magnetite by magnetic methods [2,3]. This is far more magnetite than is normally found in C2 chondrites and is slightly higher than the levels found in C1 chondrites [3]. McSween and Richardson [41 measured the bulk composition of "matrix" in many carbonaceous chondrites (including Bells) by broad beam electron microprobe analysis. They found a range of compositions among the phyllosilicate matrices of C1 and C2 chond- rites. Bells and the C1 chondrites are at the Fe-poor, Mg- and Si-rich end of this range. McSween [5] point-counted many C2 chondrites and found Bells to be among the most matrix-rich of them. He found a correlation between the composition of matrix and vol% matrix and suggested that increasing degree of alteration of chondrules and inclusions leads to increasing matrix fraction and decreasing Fe content of the matrix. According to his model, Bells should be among the most heavily altered of the C2 chondrites. Until recently, most of the recovered samples of Bells have been in the private collection of Mr. Oscar Monnig and the only sample available for pet- rographic study has been a tiny thin section. -

Meteor Impact Craters
METEOROIDS, METEORITES and IMPACT CRATERS Dr. Ali Ait-Kaci, [email protected] TERMINOLOGY Rocky, iron or icy debris flying in space, 1 m to 100’s km A small asteroid, from microns to few meters. Annual events Light emitted by a meteoroid in the atmosphere A meteor brighter than Venus Light emitted by a large meteoroid as it explodes A fragment of a meteoroid that In the atmosphere survives passage through the atmosphere and hits the ground CLASSIFICATION OF METEORITES NON-DIFFERENTIATED DIFFERENTIATED CHONDRITES Stony d = 3 to 3.7 g/cm3 86 % ACHONDRITES STONY-IRON IRON Stony Iron-Stony Fe/Ni alloy d = 2.8 to 3.1 g/cm3 d = 4.3 to 4.8 g/cm3 d = 7 to 8 g/cm3 8 % 1 % 5 % NON-DIFFERENTIATED METEORITES : CHONDRITES ORDINARY CARBONACEOUS ENSTATITE RUMURUTI KAKANGARI H CB EH CH L EL CK LL CM CR CV CO CI NON-DIFFERENTIATED METEORITES : CHONDRITES Chondrites are stony meteorites, named the presence of small spherical bodies, about 1 mm in diameter named chondrules. From their shapes and the texture of the crystals in them, chondrules appear to have been free-floating molten droplets in the solar nebula. They are typically about 4,566.6 ± 1.0 By old, which is Olivine Chondrule Olivine+ Pyroxene then dating the formation of Chondrule the Solar System itself. Chondrites are thought to represent material from the Solar System that never coalesced into large bodies. Chondritic asteroids are some of the oldest and most primitive materials in the solar system. Other Components : - Refractory inclusions (including Ca-Al) - Particles rich in metallic Fe-Ni and sulfides - Isolated grains of silicate minerals - A matrix of fine-grained ( μm or less) dust - Presolar grains ORDINARY CHONDRITES : 87% Ordinary Chondrites are thought to have originated from three parent asteroids within the Asteroid Belt, between Mars and Jupiter : 6 Hebe, 243 Ida and 3628 Boznemcova. -

PDF Linkchapter
Index [Italic page numbers indicate major references] A arsenic, 116, 143, 168 brecciation, shock, 225, 231 Ashanti crater, Ghana. See Bosumtwi Brent crater, Ontario, 321 Abitibi Subprovince, 305 crater, Ghana bromine, 137 Acraman depression, South Australia, A thy ris Broodkop Shear Zone, 180 211, 212, 218 gurdoni transversalis, 114 Budevska crater, Venus, 24 geochemistry, 216 hunanensis, 114 Bunyeroo Formation, 209, 210, 219, geochronology, 219 aubrite, 145 220, 221, 222 melt rock, 216, 218 augite, 159 Bushveld layered intrusion, 337 paleomagnetism, 219 Australasian strewn field, 114, 133, See also Acraman impact structure 134, 137, 138, 139, 140, 143, Acraman impact structure, South C 144, 146 Australia, 209 australite, 136, 141, 145, 146 Cabin-Medicine Lodge thrust system, Adelaid Geosyncline, 210, 220, 222 Austria, moldavites, 142 227 adularía, 167 Cabin thrust plate, 173, 225, 226, Aeneas on Dione crater, Earth, 24 227, 231, 232 aerodynamically shaped tektites, 135 B calcite, 112, 166 Al Umchaimin depression, western Barbados, tektites, 134, 139, 142, 144 calcium, 115, 116, 128 Iraq, 259 barium, 116, 169, 216 calcium oxide, 186, 203, 216 albite, 209 Barrymore crater, Venus, 44 Callisto, rings, 30 Algoman granites, 293 Basal Member, Onaping Formation, Cambodia, circular structure, 140, 141 alkali, 156, 159, 167 266, 267, 268, 271, 272, 273, Cambrian, Beaverhead impact alkali feldspar, 121, 123, 127, 211 289, 290, 295, 296, 299, 304, structure, Montana, 163, 225, 232 almandine-spessartite, 200 307, 308, 310, 311, 314 Canadian Arctic, -

57Fe Mossbauer Study of Tektites BJ Evans and LX Leung C-.) University
57Fe Mossbauer Study of Tektites r' B. J. Evans and L. X. Leung C-.) Department of Chemistry -rn University of Michigan C Ann Arbor, MI 48109 N176-31121 (NASA-CR-148774) Fe-57 MOESSBAUEB STUDY OF TEKTITES Final Report (Michigan Univ.) 55 p HC $4.50 CSCI 03B Unclas G3/91 50391 SE ABSTRACT 57 i Fe Mossbauer measurements have been made selected moldavite, australite, philippinite and georgia tektites. The spectra consist of two apparent lines but at least two quadru pole doublets can be fitted to these spectra.- The Mossbauer parameters for these doublets indicate that they arise from Fe2 + ions with local environments, which are relatively rich and relatively poor in calcium, respectively, similar to those in clinopyroxenes. No evidence for Fe3+/Fe 2 + ratios above 0.01 (estimated detection limit) have been found in any tektite. Tektites are considerably more reduced than previously believed and the extent of the reduction shows little or no variation among different types of tlktites. These results limit the source materials of tektites to minerals in which the iron is uniformly highly reduced and in which the iron is contained clinopyroxene-like phases. I. INTRODUCTION The origin and source materials of tektites continue to be a conundrum. On the one hand, there is agreement among some inves tigators on their connascence with terrestrial meteoritic impact events while, on the other hand, there is a concensus among a smaller, more active group of tektite investigators that tektites are lunar materials being either the ejecta from a lunar meteoritic impact or lunar volcanism.11 In his recent monograph on tektites O'Keefe has given a well balanced review of the different theories on the origins of tektites and their strengths and shortcomings in the light of the experimental evidence. -

Intercom Debates Image, Tuition Hikes Students Voice Concerns
'TALL TALE' STAR MEASURES UP - PAGE 4 TCU DAILY SKIFF FRIDAY, MARCH 24,1995 TEXAS CHRISTIAN UNIVERSITY, FORT WORTH, TEXAS 92ND YEAR, NO. 90 Intercom debates image, tuition hikes Students voice concerns . - with Board of Trustees BY GINGER RICHARDSON "1 have a daughter here and would TCU DAILY SKIFF rather not see tuition increase to that extent," Scharbauer said. "1 think if Finding ways to improve the uni- we market ourselves as being as good versity's image while keeping tuition as Tulane, Vanderbilt or Wake Forest and fees at a reasonable level was the and then say, 'Oh, by the way, we're main issue of concern at Thursday's also $ 10,000 to $ 15,000 cheaper than f meeting between Intercom and mem- those schools,' that will be a draw to bers of the Board of Trustees. students." Student leaders and the Intercom members also asked the P» Trustee/Student Relations Commit- trustees to consider allocating funds ' j^J^jiiM^R*". tee also discussed what the financial for additional faculty, greater handi- ^^n . priorities of the university should be cap accessibility, residence hall ren- ^^^■^F # m. 'Mi,'. over the course of the next several ovations and increased scholarships years. — all of which would help improve Louis H. Barnett. a trustee from TCU's image, they said. Fort Worth, proposed increasing "The main thing we are concerned tuition to the level of schools such as about is image," said Scott Wheatley. ''*,'//'uM/t^^ i^k Tulane or Wake Forest to solve what a junior political science major and ™ %Jlmh some trustees and Intercom members student body president.