(Slc15a1 and Slc22a5) in MICE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

MAP17's Up-Regulation, a Crosspoint in Cancer and Inflammatory
García-Heredia and Carnero Molecular Cancer (2018) 17:80 https://doi.org/10.1186/s12943-018-0828-7 REVIEW Open Access Dr. Jekyll and Mr. Hyde: MAP17’s up- regulation, a crosspoint in cancer and inflammatory diseases José M. García-Heredia1,2,3 and Amancio Carnero1,3* Abstract Inflammation is a common defensive response that is activated after different harmful stimuli. This chronic, or pathological, inflammation is also one of the causes of neoplastic transformation and cancer development. MAP17 is a small protein localized to membranes with a restricted pattern of expression in adult tissues. However, its expression is common in destabilized cells, as it is overexpressed both in inflammatory diseases and in cancer. MAP17 is overexpressed in most, if not all, carcinomas and in many tumors of mesenchymal origin, and correlates with higher grade and poorly differentiated tumors. This overexpression drives deep changes in cell homeostasis including increased oxidative stress, deregulation of signaling pathways and increased growth rates. Importantly, MAP17 is associated in tumors with inflammatory cells infiltration, not only in cancer but in various inflammatory diseases such as Barret’s esophagus, lupus, Crohn’s, psoriasis and COPD. Furthermore, MAP17 also modifies the expression of genes connected to inflammation, showing a clear induction of the inflammatory profile. Since MAP17 appears highly correlated with the infiltration of inflammatory cells in cancer, is MAP17 overexpression an important cellular event connecting tumorigenesis and inflammation? Keywords: MAP17, Cancer, Inflammatory diseases Background process ends when the activated cells undergo apoptosis in Inflammatory response is a common defensive process acti- a highly regulated process that finishes after pathogens and vated after different harmful stimuli, constituting a highly cell debris have been phagocytized [9]. -
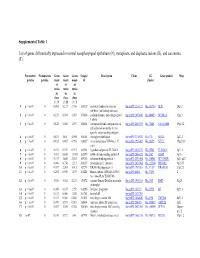
Supplemental Table 1 List of Genes Differentially Expressed In
Supplemental Table 1 List of genes differentially expressed in normal nasopharyngeal epithelium (N), metaplastic and displastic lesions (R), and carcinoma (T). Parametric Permutation Geom Geom Geom Unique Description Clone UG Gene symbol Map p-value p-value mean mean mean id cluster of of of ratios ratios ratios in in in class class class 1 : N 2 : R 3 : T 1 p < 1e-07 0 0.061 0.123 2.708 169329 secretory leukocyte protease IncytePD:2510171 Hs.251754 SLPI 20q12 inhibitor (antileukoproteinase) 2 p < 1e-07 0 0.125 0.394 1.863 163628 sodium channel, nonvoltage-gated IncytePD:1453049 Hs.446415 SCNN1A 12p13 1 alpha 3 p < 1e-07 0 0.122 0.046 1.497 160401 carcinoembryonic antigen-related IncytePD:2060355 Hs.73848 CEACAM6 19q13.2 cell adhesion molecule 6 (non- specific cross reacting antigen) 4 p < 1e-07 0 0.675 1.64 5.594 165101 monoglyceride lipase IncytePD:2174920 Hs.6721 MGLL 3q21.3 5 p < 1e-07 0 0.182 0.487 0.998 166827 nei endonuclease VIII-like 1 (E. IncytePD:1926409 Hs.28355 NEIL1 15q22.33 coli) 6 p < 1e-07 0 0.194 0.339 0.915 162931 hypothetical protein FLJ22418 IncytePD:2816379 Hs.36563 FLJ22418 1p11.1 7 p < 1e-07 0 1.313 0.645 13.593 162399 S100 calcium binding protein P IncytePD:2060823 Hs.2962 S100P 4p16 8 p < 1e-07 0 0.157 1.445 2.563 169315 selenium binding protein 1 IncytePD:2591494 Hs.334841 SELENBP1 1q21-q22 9 p < 1e-07 0 0.046 0.738 1.213 160115 prominin-like 1 (mouse) IncytePD:2070568 Hs.112360 PROML1 4p15.33 10 p < 1e-07 0 0.787 2.264 3.013 167294 HRAS-like suppressor 3 IncytePD:1969263 Hs.37189 HRASLS3 11q12.3 11 p < 1e-07 0 0.292 0.539 1.493 168221 Homo sapiens cDNA FLJ13510 IncytePD:64451 Hs.37896 2 fis, clone PLACE1005146. -

Prenatal Detection of TAR Syndrome in a Fetus with Compound Inheritance of an RBM8A SNP and a 334‑Kb Deletion: a Case Report
MOLECULAR MEDICINE REPORTS 9: 163-165, 2014 Prenatal detection of TAR syndrome in a fetus with compound inheritance of an RBM8A SNP and a 334‑kb deletion: A case report IOANNIS PAPOULIDIS1, EIRINI OIKONOMIDOU1, SANDRO ORRU2, ELISAVET SIOMOU1, MARIA KONTODIOU1, MAKARIOS ELEFTHERIADES3, VASILIOS BACOULAS4, JUAN C. CIGUDOSA5, JAVIER SUELA5, LORETTA THOMAIDIS6 and EMMANOUIL MANOLAKOS1,2 1Laboratory of Genetics, Eurogenetica S.A., Thessaloniki 55133, Greece; 2Department of Medical Genetics, University of Cagliari, Binaghi Hospital, Cagliari I‑09126, Italy; 3Embryocare, Fetal Medicine Unit, Athens 11522; 4Fetal Medicine Centre, Athens 10674, Greece; 5NIMGenetics, Madrid 28049, Spain; 6Department of Pediatrics, Aglaia Kyriakou Children's Hospital, University of Athens, Athens 11527, Greece Received May 30, 2013; Accepted October 16, 2013 DOI: 10.3892/mmr.2013.1788 Abstract. Thrombocytopenia-absent radius syndrome identified the presence of a minimally deleted 200‑kb region (TAR) is a rare genetic disorder that is characterized by the at chromosome band 1q21.1 in patients with TAR, but it is not absence of the radius bone in each forearm and a markedly sufficient to cause the phenotype (3,4). A study identified two reduced platelet count that results in life-threatening bleeding rare single nucleotide polymorphisms (SNPs) in the regulatory episodes (thrombocytopenia). Tar syndrome has been associ- region of the RBM8A gene that are involved in TAR syndrome ated with a deletion of a segment of 1q21.1 cytoband. The through the reduction of the expression of the RBM8A-encoded 1q21.1 deletion syndrome phenotype includes Tar and other Y14 protein (4). The first allele (rs139428292 G>A), which is features such as mental retardation, autism and microcephaly. -

Supplementary Table S1. List of Differentially Expressed
Supplementary table S1. List of differentially expressed transcripts (FDR adjusted p‐value < 0.05 and −1.4 ≤ FC ≥1.4). 1 ID Symbol Entrez Gene Name Adj. p‐Value Log2 FC 214895_s_at ADAM10 ADAM metallopeptidase domain 10 3,11E‐05 −1,400 205997_at ADAM28 ADAM metallopeptidase domain 28 6,57E‐05 −1,400 220606_s_at ADPRM ADP‐ribose/CDP‐alcohol diphosphatase, manganese dependent 6,50E‐06 −1,430 217410_at AGRN agrin 2,34E‐10 1,420 212980_at AHSA2P activator of HSP90 ATPase homolog 2, pseudogene 6,44E‐06 −1,920 219672_at AHSP alpha hemoglobin stabilizing protein 7,27E‐05 2,330 aminoacyl tRNA synthetase complex interacting multifunctional 202541_at AIMP1 4,91E‐06 −1,830 protein 1 210269_s_at AKAP17A A‐kinase anchoring protein 17A 2,64E‐10 −1,560 211560_s_at ALAS2 5ʹ‐aminolevulinate synthase 2 4,28E‐06 3,560 212224_at ALDH1A1 aldehyde dehydrogenase 1 family member A1 8,93E‐04 −1,400 205583_s_at ALG13 ALG13 UDP‐N‐acetylglucosaminyltransferase subunit 9,50E‐07 −1,430 207206_s_at ALOX12 arachidonate 12‐lipoxygenase, 12S type 4,76E‐05 1,630 AMY1C (includes 208498_s_at amylase alpha 1C 3,83E‐05 −1,700 others) 201043_s_at ANP32A acidic nuclear phosphoprotein 32 family member A 5,61E‐09 −1,760 202888_s_at ANPEP alanyl aminopeptidase, membrane 7,40E‐04 −1,600 221013_s_at APOL2 apolipoprotein L2 6,57E‐11 1,600 219094_at ARMC8 armadillo repeat containing 8 3,47E‐08 −1,710 207798_s_at ATXN2L ataxin 2 like 2,16E‐07 −1,410 215990_s_at BCL6 BCL6 transcription repressor 1,74E‐07 −1,700 200776_s_at BZW1 basic leucine zipper and W2 domains 1 1,09E‐06 −1,570 222309_at -

The Genetic Architecture of Fasting Plasma Triglyceride Response to Fenofibrate Treatment
European Journal of Human Genetics (2008) 16, 603–613 & 2008 Nature Publishing Group All rights reserved 1018-4813/08 $30.00 www.nature.com/ejhg ARTICLE The genetic architecture of fasting plasma triglyceride response to fenofibrate treatment Jennifer A Smith*,1, Donna K Arnett2, Reagan J Kelly1, Jose M Ordovas3, Yan V Sun1, Paul N Hopkins4,JamesEHixson5,RobertJStraka6, James M Peacock7 and Sharon LR Kardia1 1Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, USA; 2Department of Epidemiology, School of Public Health, University of Alabama at Birmingham, Birmingham, AL, USA; 3Nutrition and Genomics Laboratory, Jean Mayer-United States Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, Boston, MA, USA; 4Cardiovascular Genetics Research, University of Utah, Salt Lake City, UT, USA; 5Human Genetics Center, University of Texas Health Science, Houston, TX, USA; 6Department of Experimental and Clinical Pharmacology, College of Pharmacy, University of Minnesota, Minneapolis, MN, USA; 7Department of Epidemiology, School of Public Health, University of Minnesota, Minneapolis, MN, USA Metabolic response to the triglyceride (TG)-lowering drug, fenofibrate, is shaped by interactions between genetic and environmental factors, yet knowledge regarding the genetic determinants of this response is primarily limited to single-gene effects. Since very low-density lipoprotein (VLDL) is the central carrier of fasting TG, identifying factors that affect both total TG and VLDL–TG response to fenofibrate is critical for predicting individual fenofibrate response. As part of the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study, 688 individuals from 161 families were genotyped for 91 single-nucleotide polymorphisms (SNPs) in 25 genes known to be involved in lipoprotein metabolism. -

Common Variant of PDZ Domain Containing 1 (PDZK1) Gene Is Associated with Gout Susceptibility: a Replication Study and Meta-Analysis in Japanese Population
Drug Metabolism and Pharmacokinetics xxx (2016) 1e3 Contents lists available at ScienceDirect Drug Metabolism and Pharmacokinetics journal homepage: http://www.journals.elsevier.com/drug-metabolism-and- pharmacokinetics Note Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: A replication study and meta-analysis in Japanese population * Toshihide Higashino a, 1, Hirotaka Matsuo a, , 1, Masayuki Sakiyama a, Akiyoshi Nakayama a, Takahiro Nakamura b, Tappei Takada c, Hiraku Ogata a, Yusuke Kawamura a, Makoto Kawaguchi a, Mariko Naito d, Sayo Kawai d, Yuzo Takada e, Hiroshi Ooyama f, Hiroshi Suzuki c, Nariyoshi Shinomiya a a Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, Tokorozawa, Japan b Laboratory of Mathematics, National Defense Medical College, Tokorozawa, Japan c Department of Pharmacy, The University of Tokyo Hospital, Faculty of Medicine, The University of Tokyo, Tokyo, Japan d Department of Preventive Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan e Faculty of Medical Science, Teikyo University of Science, Tokyo, Japan f Ryougoku East Gate Clinic, Tokyo, Japan article info abstract Article history: PDZ domain containing 1 (PDZK1) is a scaffold protein that organizes a transportsome and regulates Received 24 May 2016 several transporters' functions including urate and drug transporters. Therefore, PDZK1 in renal proximal Received in revised form tubules may affect serum uric acid levels through PDZK1-binding renal urate transporters. Two previous 4 July 2016 studies in Japanese male population reported that a PDZK1 single nucleotide polymorphism (SNP), Accepted 25 July 2016 rs12129861, was not associated with gout. In the present study, we performed a further association Available online xxx analysis between gout and rs12129861 in a different large-scale Japanese male population and a meta- analysis with previous Japanese population studies. -

Cardiac Camp-PKA Signaling Compartmentalization in Myocardial Infarction
cells Review Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Infarction Anne-Sophie Colombe and Guillaume Pidoux * INSERM, UMR-S 1180, Signalisation et Physiopathologie Cardiovasculaire, Université Paris-Saclay, 92296 Châtenay-Malabry, France; [email protected] * Correspondence: [email protected] Abstract: Under physiological conditions, cAMP signaling plays a key role in the regulation of car- diac function. Activation of this intracellular signaling pathway mirrors cardiomyocyte adaptation to various extracellular stimuli. Extracellular ligand binding to seven-transmembrane receptors (also known as GPCRs) with G proteins and adenylyl cyclases (ACs) modulate the intracellular cAMP content. Subsequently, this second messenger triggers activation of specific intracellular downstream effectors that ensure a proper cellular response. Therefore, it is essential for the cell to keep the cAMP signaling highly regulated in space and time. The temporal regulation depends on the activity of ACs and phosphodiesterases. By scaffolding key components of the cAMP signaling machinery, A-kinase anchoring proteins (AKAPs) coordinate both the spatial and temporal regulation. Myocar- dial infarction is one of the major causes of death in industrialized countries and is characterized by a prolonged cardiac ischemia. This leads to irreversible cardiomyocyte death and impairs cardiac function. Regardless of its causes, a chronic activation of cardiac cAMP signaling is established to compensate this loss. While this adaptation is primarily beneficial for contractile function, it turns out, in the long run, to be deleterious. This review compiles current knowledge about cardiac cAMP compartmentalization under physiological conditions and post-myocardial infarction when it Citation: Colombe, A.-S.; Pidoux, G. appears to be profoundly impaired. Cardiac cAMP-PKA Signaling Compartmentalization in Myocardial Keywords: heart; myocardial infarction; cardiomyocytes; cAMP signaling; A-kinase anchoring Infarction. -

Soluble Uric Acid Increases PDZK1 and ABCG2 Expression in Human
Chen et al. Arthritis Research & Therapy (2018) 20:20 DOI 10.1186/s13075-018-1512-4 RESEARCHARTICLE Open Access Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway Mo Chen1,2†, Xiaoyong Lu1†,CiLu1†, Ning Shen3, Yujie Jiang1, Menglu Chen1 and Huaxiang Wu1* Abstract Background: In addition to the kidney, the intestine is one of the most important organs involved in uric acid excretion. However, the mechanism of urate excretion in the intestine remains unclear. Therefore, the relationship between soluble uric acid and the gut excretion in human intestinal cells was explored. The relevant signaling molecules were then also examined. Methods: HT-29 and Caco-2 cell lines were stimulated with soluble uric acid. Western blotting and qRT-PCR were used to measure protein and mRNA levels. Subcellular fractionation methods and immunofluorescence were used to quantify the proteins in different subcellular compartments. Flow cytometry experiments examined the function of ATP-binding cassette transporter, subfamily G, member 2 (ABCG2). Small interfering RNA transfection was used to assess the interaction between ABCG2 and PDZ domain-containing 1 (PDZK1). Results: Soluble uric acid increased the expression of PDZK1 and ABCG2. The stimulation of soluble uric acid also facilitated the translocation of ABCG2 from the intracellular compartment to the plasma membrane and increased its transport activity. Moreover, the upregulation of PDZK1 and ABCG2 by soluble uric acid was partially decreased by either TLR4-NLRP3 inflammasome inhibitors or PI3K/Akt signaling inhibitors. Furthermore, PDZK1 knockdown significantly inhibited the expression and transport activity of ABCG2 regardless of the activation by soluble uric acid, demonstrating a pivotal role for PDZK1 in the regulation of ABCG2. -

(ASD) and Intellectual Disability
ndrom Sy es tic & e G n Mafalda et al., J Genet Syndr Gene Ther 2016, 7:5 e e n G e f T o Journal of Genetic Syndromes & DOI: 10.4172/2157-7412.1000307 h l e a r n a r p u y o J ISSN: 2157-7412 Gene Therapy Research Article Open Access A Genome Wide Copy Number Variations Analysis in Autism Spectrum Disorder (ASD) and Intellectual Disability (ID) in Italian Families Mucciolo Mafalda1, Chiara Di Marco1,3, Roberto Canitano2, Sabrina Buoni2, Elisa Frullanti1, Maria Antonietta Mencarelli13, Bizzarri Veronica1, Sonia Amabile1, Lucia Radice2, Margherita Baldassarri1,3, Caterina Lo Rizzo13, Ilaria Meloni1, Joussef Hayek2, Alessandra Renieri1,3 and Francesca Mari1,3 1Medical Genetics-Department of Biotechnology, University of Siena-Policlinico S. Maria alle Scotte, 53100 Siena, Italy 2Department of Child Neuropsychiatry, University Hospital of Siena, 53100 Siena, Italy 3Department of Medical Genetics, Azienda Ospedaliera Universitaria Senese, Siena, Italy Abstract Background: Autism Spectrum Disorders (ASD) and Intellectual Disability (ID) represent lifelong conditions with severe impact on behavior and lifestyle of patients and their families. Array comparative genomic hybridization (array- CGH) has clarified the underlying genetic causes of ASD and ID by CNVs identification in several chromosomal regions with susceptibility to different levels of severity of ASD or ID in up to 1% of patients. Methods: Using oligo array-CGH we analyzed 476 unrelated subjects with ASD or ID, thoroughly investigated by both child neuropsychiatrists and clinical geneticists. The inheritance of the CNV was tested in the majority of cases (82% of positive cases). Results: A total of 198 rearrangements were identified in 154 cases. -

Dissecting Molecular Genetic Mechanisms of 1Q21.1 CNV in Neuropsychiatric Disorders
International Journal of Molecular Sciences Review Dissecting Molecular Genetic Mechanisms of 1q21.1 CNV in Neuropsychiatric Disorders Joy Yoon and Yingwei Mao * Department of Biology, Eberly College of Science, Pennsylvania State University, University Park, PA 16802, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-814-867-4739 Abstract: Pathogenic copy number variations (CNVs) contribute to the etiology of neurodevelopmen- tal/neuropsychiatric disorders (NDs). Increased CNV burden has been found to be critically involved in NDs compared with controls in clinical studies. The 1q21.1 CNVs, rare and large chromosomal microduplications and microdeletions, are detected in many patients with NDs. Phenotypes of duplication and deletion appear at the two ends of the spectrum. Microdeletions are predominant in individuals with schizophrenia (SCZ) and microcephaly, whereas microduplications are predominant in individuals with autism spectrum disorder (ASD) and macrocephaly. However, its complexity hinders the discovery of molecular pathways and phenotypic networks. In this review, we summarize the recent genome-wide association studies (GWASs) that have identified candidate genes positively correlated with 1q21.1 CNVs, which are likely to contribute to abnormal phenotypes in carriers. We discuss the clinical data implicated in the 1q21.1 genetic structure that is strongly associated with neurodevelopmental dysfunctions like cognitive impairment and reduced synaptic plasticity. We further present variations reported in the phenotypic severity, genomic penetrance and inheritance. Keywords: copy number variation; microdeletion; microduplication; schizophrenia; autism spectrum Citation: Yoon, J.; Mao, Y. Dissecting disorder; microcephaly; macrocephaly; neurodegeneration; synaptic plasticity Molecular Genetic Mechanisms of 1q21.1 CNV in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2021, 22, 5811. https://doi.org/10.3390/ 1. -

(PDZK1) Gene Is Associated with Gout Susceptibility: a Replication Study and Meta-Analysis in Japanese Population
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Drug Metabolism and Pharmacokinetics 31 (2016) 464e466 Contents lists available at ScienceDirect Drug Metabolism and Pharmacokinetics journal homepage: http://www.journals.elsevier.com/drug-metabolism-and- pharmacokinetics Note Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: A replication study and meta-analysis in Japanese population * Toshihide Higashino a, 1, Hirotaka Matsuo a, , 1, Masayuki Sakiyama a, Akiyoshi Nakayama a, Takahiro Nakamura b, Tappei Takada c, Hiraku Ogata a, Yusuke Kawamura a, Makoto Kawaguchi a, Mariko Naito d, Sayo Kawai d, Yuzo Takada e, Hiroshi Ooyama f, Hiroshi Suzuki c, Nariyoshi Shinomiya a a Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, Tokorozawa, Japan b Laboratory of Mathematics, National Defense Medical College, Tokorozawa, Japan c Department of Pharmacy, The University of Tokyo Hospital, Faculty of Medicine, The University of Tokyo, Tokyo, Japan d Department of Preventive Medicine, Nagoya University Graduate School of Medicine, Nagoya, Japan e Faculty of Medical Science, Teikyo University of Science, Tokyo, Japan f Ryougoku East Gate Clinic, Tokyo, Japan article info abstract Article history: PDZ domain containing 1 (PDZK1) is a scaffold protein that organizes a transportsome and regulates Received 24 May 2016 several transporters' functions including urate and drug transporters. Therefore, PDZK1 in renal proximal Received in revised form tubules may affect serum uric acid levels through PDZK1-binding renal urate transporters. Two previous 4 July 2016 studies in Japanese male population reported that a PDZK1 single nucleotide polymorphism (SNP), Accepted 25 July 2016 rs12129861, was not associated with gout.