Ethnobiology of Georgia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Length-Weight Relationships of Two Clupeonella Species (Clupeidae) from Northwestern Turkey
Turkish Journal of Bioscience and Collections Volume 4, Number 1, 2020 E-ISSN: 2601-4292 SHORT COMMUNICATION/KISA BİLDİRİ Length-Weight Relationships of two Clupeonella species (Clupeidae) from Northwestern Turkey Kemal Aydoğan1 , Müfit Özuluğ2 Abstract The length-weight relationships (LWRs) of Clupeonella cultriventris and Clupeonella muhlisi were analysed. Fish samples were collected gill nets (10 mm mesh sized) from the Büyükçekmece Reservoir and Küçükçekmece Lagoon April and June 2016. Samples from 1İstanbul University, Institute of Sciences, Durusu Reservoir and Uluabat Lake were obtained from Istanbul University Science İstanbul, Turkey Faculty Hydrobiology Museum. The values of parameter b in the LWR equations varied 2 İstanbul University, Faculty of Science, from 3.177 (Küçükçekmece Lake population) to 3.496 (Büyükçekmece Reservoir Department of Biology, İstanbul, Turkey population) for C. cultriventris and 3.258 (Uluabat Lake) for C. muhlisi. ORCID: K.A. 0000-0003-3381-8549; Keywords: Length-weight relationship, Clupeonella, Uluabat Lake, İstanbul M.Ö. 0000-0002-1437-3890 Received: 18.02.2020 Revision Requested: 26.02.2020 Last Revision Received: 05.03.2020 Accepted: 06.03.2020 Correspondence: Kemal Aydoğan [email protected] Citation: Aydoğan, K., & Özuluğ, M. (2020). Length-Weight relationships of two Clupeonella species (Clupeidae) from Northwestern Turkey. Turkish Journal of Bioscience and Collections, 4(1), 27–29. https://doi.org/10.26650/tjbc.20200012 Introduction Materials and Methods There are three species of genus Clupeonella in the Black In this study all materials belong to Uluabat Lake, Sea basin, Clupeonella abrau (Maliatsky, 1930), Büyükçekmece and Durusu Reservoirs and Clupeonella cultriventris (Nordmann, 1840) and Küçükçekmece Lagoon. Uluabat Lake is a large and very Clupeonaella muhlisi Neu, 1934 (Frose & Pauly, 2020). -

How to Cite Complete Issue More Information About This Article
Cuadernos de Investigación UNED ISSN: 1659-4266 ISSN: 1659-4266 Universidad Estatal a Distancia de Costa Rica Aliasghari, Mehrdad; AnvariFar, Hossein; Iqbal Mir, Javaid Effects of fishing and natural factors on the population of the fish Clupeonella grimmi (Clupeiformes: Clupeidae) in southern waters of the Caspian Sea Cuadernos de Investigación UNED, vol. 9, no. 1, 2017, pp. 185-191 Universidad Estatal a Distancia de Costa Rica Available in: https://www.redalyc.org/articulo.oa?id=515653587025 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative Effects of fishing and natural factors on the population of the fish Clupeonella grimmi (Clupeiformes: Clupeidae) in southern waters of the Caspian Sea Mehrdad Aliasghari1, Hossein AnvariFar2 & Javaid Iqbal Mir3* 1. Young Researchers and Elite Club, Qaemshahr branch, Islamic Azad University, Qaemshahr, Iran; [email protected] 2. Department of Fisheries, Faculty of Animal Science and Fisheries, University of Agriculture and Natural Resources, P.O. Box 578, Sari, Iran; [email protected] 3. Directorate of Coldwater Fisheries Research, (ICAR), Bhimtal-263136, Nainital, Uttarakhand, India, [email protected] * Corresponding author: [email protected] Received 18-VIII-2016 • Corrected 25-I-2017 • Accepted 01-II-2017 ABSTRACT: The present study aimed to investigate the changes of big- RESUMEN: Efecto de la pesca y los parámetros naturales en la po- eye kilka Clupeonella grimmi population caused by human and natural blación de Clupeonella grimmi (Clupeiformes: Clupeidae) en las factors in southern waters of the Caspian Sea. -
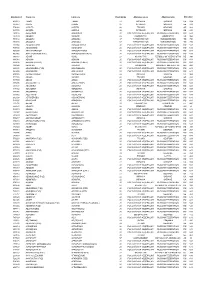
FÁK Állomáskódok
Állomáskód Orosz név Latin név Vasút kódja Államnév orosz Államnév latin Államkód 406513 1 МАЯ 1 MAIA 22 УКРАИНА UKRAINE UA 804 085827 ААКРЕ AAKRE 26 ЭСТОНИЯ ESTONIA EE 233 574066 ААПСТА AAPSTA 28 ГРУЗИЯ GEORGIA GE 268 085780 ААРДЛА AARDLA 26 ЭСТОНИЯ ESTONIA EE 233 269116 АБАБКОВО ABABKOVO 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 737139 АБАДАН ABADAN 29 УЗБЕКИСТАН UZBEKISTAN UZ 860 753112 АБАДАН-I ABADAN-I 67 ТУРКМЕНИСТАН TURKMENISTAN TM 795 753108 АБАДАН-II ABADAN-II 67 ТУРКМЕНИСТАН TURKMENISTAN TM 795 535004 АБАДЗЕХСКАЯ ABADZEHSKAIA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 795736 АБАЕВСКИЙ ABAEVSKII 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 864300 АБАГУР-ЛЕСНОЙ ABAGUR-LESNOI 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 865065 АБАГУРОВСКИЙ (РЗД) ABAGUROVSKII (RZD) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 699767 АБАИЛ ABAIL 27 КАЗАХСТАН REPUBLIC OF KAZAKHSTAN KZ 398 888004 АБАКАН ABAKAN 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 888108 АБАКАН (ПЕРЕВ.) ABAKAN (PEREV.) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 398904 АБАКЛИЯ ABAKLIIA 23 МОЛДАВИЯ MOLDOVA, REPUBLIC OF MD 498 889401 АБАКУМОВКА (РЗД) ABAKUMOVKA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 882309 АБАЛАКОВО ABALAKOVO 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 408006 АБАМЕЛИКОВО ABAMELIKOVO 22 УКРАИНА UKRAINE UA 804 571706 АБАША ABASHA 28 ГРУЗИЯ GEORGIA GE 268 887500 АБАЗА ABAZA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 887406 АБАЗА (ЭКСП.) ABAZA (EKSP.) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 -

Peasant Oaths, Furious Icons and the Quest for Agency: Tracing
15 praktyka teoretyczna 1(39)/2021 } LUKA NAKHUTSRISHVILI (ORCID: 0000-0002-5264-0064) Peasant Oaths, Furious Icons and the Quest for Agency: Tracing Subaltern Politics in Tsarist Georgia on the Eve of the 1905 Revolution Part I: The Prose of the Intelligentsia and Its Peasant Symptoms This two-part transdisciplinary article elaborates on the autobiographical account of the Georgian Social-Democrat Grigol Uratadze regarding the oath pledged by protesting peasants from Guria in 1902. The oath inaugurated their mobilization in Tsarist Georgia in 1902, culminating in full peasant self-rule in the “Gurian Republic” by 1905. The study aims at a historical-anthropological assessment of the asymmetries in the alliance formed by peasants and the revolutionary intelligentsia in the wake of the oath as well as the tensions that crystallized around the oath between the peasants and Tsarist officials. In trying to recover the traces of peasant politics in relation to multiple hegemonic forces in a modernizing imperial borderland, the article invites the reader to reconsider the existing assumptions about historical agency, linguistic conditions of subjectivity, and the relation- ship between politics and the material and customary dimen- sions of religion. The ultimate aim is to set the foundations for a future subaltern reading of the practices specific to the peasant politics in the later “Gurian Republic”. The first part of the article starts with a reading of Uratadze’s narration of the 1902 inaugural oath “against the grain”. Keywords: agency, intelligentsia, oath, Orthodox icons, peasantry, political the- ology, Russian Empire, secular studies, speech-act, subaltern praktyka teoretyczna 1(39)/2021 16 I.1. -

“Borderization” Continues in Georgia
EURASIA “Borderization” Continues in Georgia OE Watch Commentary: In August 2019, the Georgian government established a police checkpoint near the village of Chorchana as part of an effort to counter what it has called the “borderization” of its territory by forces from the occupied region of South Ossetia (see: “A Change in the ‘Borderization’ Process for Georgia?” OE Watch, October 2019). The checkpoint initially appeared to have some effect and helped the Georgian government with public relations at a time when many in the country did not believe enough was being done to counter “borderization.” As the accompanying excerpted articles report, Georgia’s border with the occupied South Ossetia region continues to shift and the reported incidents provide an update on how this is taking place. The articles, from Georgia’s English-language news website Civil.ge, report on two recent incidents in which occupying forces erected “illegal A Georgian villager is left beyond the barbwire installed by the Russian troops along the South Ossetia-Georgia contact line in September 2013. installations” on Georgian government controlled territory. The first incident Source: VOA via Wikimedia, https://commons.wikimedia.org/wiki/File:Barbwires_in_South_Ossetia,_Georgia._September_2013.jpg, took place on 14 January near the village of Chorchana of the Khashuri Public domain Municipality, at the dividing line with the Tskhinvali Region. The second incident took place on 20 January in the village of Gugutiantkari of the Gori Municipality. The villages of Chorchana and Gugutiantkari are in two different districts that do not border each other, but are notable since both made headlines in August 2019 – Chorchana because of the police checkpoint and Gugutiantkari since part of it became divided after occupation forces installed fencing. -

Gambling in Georgia Second Report
Gambling in Georgia Second Report July 2015 2 Transparency International Georgia Research supervisor: Levan Natroshvili Researcher: Mariam Chachua Transparency International Georgia Address: 26 Rustaveli Avenue, Tbilisi Georgia 0108 Phone: (+995 32) 292 14 03 E-mail: [email protected] Web: http://transparency.ge The report was prepared with the financial support of the Swedish International Development Cooperation Agency (Sida). All opinions expressed herein belong to Transparency International Georgia and may not express the views of the donor. Gambling in Georgia: Second Report 3 Contents I. Summary ........................................................................................................................................................... 4 II. Introduction ..................................................................................................................................................... 7 III. Public Opinion Survey Results ........................................................................................................................ 9 IV. Gambling-Related Problems ........................................................................................................................ 13 1. Problem and underage gambling .............................................................................................................. 13 2. Money laundering ..................................................................................................................................... 14 3. Control -
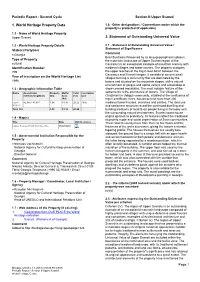
1. World Heritage Property Data 2. Statement of Outstanding Universal
Periodic Report - Second Cycle Section II-Upper Svaneti 1. World Heritage Property Data 1.8 - Other designations / Conventions under which the property is protected (if applicable) 1.1 - Name of World Heritage Property Upper Svaneti 2. Statement of Outstanding Universal Value 1.2 - World Heritage Property Details 2.1 - Statement of Outstanding Universal Value / Statement of Significance State(s) Party(ies) Comment Georgia Brief Synthesis Preserved by its long geographical isolation, Type of Property the mountain landscape of Upper Svaneti region of the cultural Caucasus is an exceptional example of mountain scenery with Identification Number medieval villages and tower houses. The property occupies 709 the upper reaches of the lnguri river basin between the Caucasus and Svaneti ranges. It consists of several small Year of inscription on the World Heritage List villages forming a community that are dominated by the 1996 towers and situated on the mountain slopes, with a natural environment of gorges and alpine valleys and a backdrop of 1.3 - Geographic Information Table snow-covered mountains. The most notable feature of the Name Coordinates Property Buffer Total Inscription settlements is the abundance of towers. The village of (latitude/longitude) (ha) zone (ha) year Chazhashi in Ushguli community, situated at the confluence of (ha) lnguri and Black rivers, has preserved more than 200 Upper 42.916 / 43.011 1.06 19.16 20.22 1996 medieval tower houses, churches and castles. The land use Svaneti and settlement structure reveal the continued dwelling and Total (ha) 1.06 19.16 20.22 building traditions of local Svan people living in harmony with the surrounding natural environment. -

Upper Svaneti Adaptation Strategy to the Climate Change
Upper Svaneti Adaptation Strategy to the Climate Change Tbilisi 2014 1 The present report is drafted in the process of preparation of Georgia’s Third National Communication to the UNFCCC. The preparation process involved a large group of specialists, representing: the Ministry of Environment and National Resources Protection of Georgia; the Ministry of Agriculture of Georgia; the Ministry of Energy of Georgia; the Ministry of Economy and Sustainable Development of Georgia; the Ministry of Labor, Health and Social Affairs of Georgia; the Ministry of Regional Development and Infrastructure of Georgia; the Ministry of Education and Science of Georgia; Georgian National Agency of Cultural Heritage Protection; National Environmental Agency; Institute of Geography; individual academic institutes; representatives of local government of Mestia municipality and local consultants engaged in tourism, health and agriculture, independent experts and NGOs. Published with the support of the United Nations Development Programme (UNDP) Georgia "The views expressed in this publication belong to the authors and do not necessarily reflect the opinions of the United Nations or the United Nations Development Programme“ © UNDP Georgia 2014 Copyright Published in Georgia 2 Abbreviations ADA - Austrian Development Agency CDM - Clean Development Mechanism CTCN – Climate Technology Centre and Network CVD- Cardiovascular Diseases ENVSEC -Environmental Security Initiative EU –European Union EWS – Early Warning Systems GCF - Green Climate Fund GDP –Gross Domestic -

Conferenceproceedingseng Final
CLIMATE CHANGE AT THE LOCAL LEVEL: POLICY AND ACTION National Conference April, 2016 Institutionalization of Climate Change Adaptation and Mitigation in Georgian Regions (ICCAMGR) This Publication is made possible by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of NALAG and authors of the texts and do not necessarily reflect the views of USAID or the United States Government. CONTENTS BACKGROUND INFORMATION ................................................... 6 Brief History ........................................................................................................ 6 CLIMATE CHANGE AT THE LOCAL LEVEL: POLICY AND ACTION ....... 7 CONFERENCE REPORTS .......................................................... 8 Climate Change and Agriculture ............................................................................. 9 Regional Information Consultation Centers (RICC) in Georgia .................................. 10 Impacts of Climate Change on Industry ................................................................ 10 Energy Sector Vulnerability to Climate Change ...................................................... 11 Climate Change and the Social Sector .................................................................. 11 INTERNATIONAL EXPERIENCE ................................................ 12 Mountains and Climate Change: Experience from Alps - Austria ............................. 12 Climate Plans in Western Europe: -

Pilot Integrated Regional Development Programme for Guria, Imereti, Kakheti and Racha Lechkhumi and Kvemo Svaneti 2020-2022 2019
Pilot Integrated Regional Development Programme for Guria, Imereti, Kakheti and Racha Lechkhumi and Kvemo Svaneti 2020-2022 2019 1 Table of Contents List of maps and figures......................................................................................................................3 List of tables ......................................................................................................................................3 List of Abbreviations ..........................................................................................................................4 Chapter I. Introduction – background and justification. Geographical Coverage of the Programme .....6 1.1. General background ........................................................................................................................... 6 1.2. Selection of the regions ..................................................................................................................... 8 Chapter II. Socio-economic situation and development trends in the targeted regions .........................9 Chapter ...........................................................................................................................................24 III. Summary of territorial development needs and potentials to be addressed in targeted regions .... 24 Chapter IV. Objectives and priorities of the Programme ................................................................... 27 4.1. Programming context for setting up PIRDP’s objectives and priorities .......................................... -

8 Socio-Economic Baseline WREP Sectional Replacement Project, Georgia Environmental and Social Impact Assessment Final
Chapter 8 Socio-Economic Baseline WREP Sectional Replacement Project, Georgia Environmental and Social Impact Assessment Final TABLE OF CONTENTS 8 SOCIO-ECONOMIC BASELINE ...................................................................... 8-1 8.1 Introduction ............................................................................................... 8-1 8.1.1 Approach ........................................................................................................ 8-1 8.1.2 Data Gathering ............................................................................................... 8-1 8.2 National Context ....................................................................................... 8-2 8.2.1 Data Quality Issues ......................................................................................... 8-2 8.2.2 National Background ...................................................................................... 8-3 8.2.3 Security Overview ........................................................................................... 8-3 8.2.4 National Economy ........................................................................................... 8-3 8.2.5 Transport Sector ............................................................................................. 8-5 8.2.6 Demographic Characteristics .......................................................................... 8-5 8.2.7 Ethnicity/Nationality ........................................................................................ 8-6 8.2.8 Incomes -

Review of Fisheries and Aquaculture Development Potentials in Georgia
FAO Fisheries and Aquaculture Circular No. 1055/1 REU/C1055/1(En) ISSN 2070-6065 REVIEW OF FISHERIES AND AQUACULTURE DEVELOPMENT POTENTIALS IN GEORGIA Copies of FAO publications can be requested from: Sales and Marketing Group Office of Knowledge Exchange, Research and Extension Food and Agriculture Organization of the United Nations E-mail: [email protected] Fax: +39 06 57053360 Web site: www.fao.org/icatalog/inter-e.htm FAO Fisheries and Aquaculture Circular No. 1055/1 REU/C1055/1 (En) REVIEW OF FISHERIES AND AQUACULTURE DEVELOPMENT POTENTIALS IN GEORGIA by Marina Khavtasi † Senior Specialist Department of Integrated Environmental Management and Biodiversity Ministry of the Environment Protection and Natural Resources Tbilisi, Georgia Marina Makarova Head of Division Water Resources Protection Ministry of the Environment Protection and Natural Resources Tbilisi, Georgia Irina Lomashvili Senior Specialist Department of Integrated Environmental Management and Biodiversity Ministry of the Environment Protection and Natural Resources Tbilisi, Georgia Archil Phartsvania National Consultant Thomas Moth-Poulsen Fishery Officer FAO Regional Office for Europe and Central Asia Budapest, Hungary András Woynarovich FAO Consultant FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2010 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned.