Paricalcitol Oral Formulation (Zemplar®▼)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

A New Role for Vitamin D Receptor Activation in Chronic Kidney Disease
A NEW ROLE FOR VITAMIN D RECEPTOR ACTIVATION IN CHRONIC KIDNEY DISEASE José M Valdivielso 1, Jorge Cannata-Andía2, Blai Coll 1 and Elvira Fernández 1 1Laboratorio de Nefrología Experimental, Hospital Universitario Arnau de Vilanova, IRBLLEIDA, REDinREN ISCIII Lleida, Spain. 2Servicio de Metabolismo Óseo y Mineral. Hospital Universitario Central de Asturias. Instituto “Reina Sofía” de Investigación. REDinREN ISCIII. Universidad de Oviedo Running Title: New role of VDRA Correspondence to: Dr. José M Valdivielso, Laboratorio de Nefrología Experimental, IRBLLEIDA Hospital Universitari Arnau de Vilanova Rovira Roure 80 25198 Lleida, Spain. Phone: +34 973003650 Fax: +34 973702213 e-mail: [email protected] Abstract Vitamin D has proven to be much more than a simple ‘calcium hormone’. The fact that the vitamin D receptor has been found in cells not related to mineral metabolism supports that statement. The interest of nephrologists in Vitamin D and its effects beyond mineral metabolism has increased over the last few years, evidencing the importance of this so-called ‘sunshine hormone’. In the present review, we highlight the most recent developments in the traditional use of vitamin D in chronic kidney disease (CKD) patients, namely the control of secondary hyperparathyroidism (sHPT). Furthermore, we also explore the data available regarding the new possible therapeutic uses of vitamin D for the treatment of other complications present in CKD patients, such as vascular calcification, left ventricular hypertrophy or proteinuria. Finally, some still scarce but very promising data regarding a possible role of vitamin D in kidney transplant patients are also reviewed. The available data point to a potential beneficial effect of vitamin D in CKD patients beyond the control of mineral metabolism. -
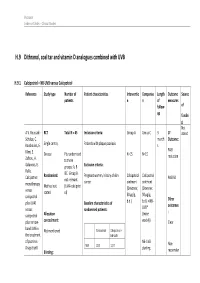
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

Paricalcitol Versus Calcitriol in the Treatment of Secondary Hyperparathyroidism
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 63 (2003), pp. 1483–1490 Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism STUART M. SPRAGUE,FRANCISCO LLACH,MICHAEL AMDAHL,CAROL TACCETTA, and DANIEL BATLLE Division of Nephrology/Hypertension and Department of Medicine, Northwestern University Feinberg School of Medicine, Evanston and Chicago, Illinois; Georgetown University Medical School, Washington, D.C.; and Abbott Laboratories, North Chicago, Illinois Paricalcitol versus calcitriol in the treatment of secondary hyper- Osteitis fibrosa cystica, resulting from poorly con- parathyroidism. trolled secondary hyperparathyroidism, remains a com- Background. Management of secondary hyperparathyroid- mon manifestation of renal osteodystrophy and causes ism has included the use of active vitamin D or vitamin D ana- logs for the suppression of parathyroid hormone (PTH) secre- significant morbidity in patients with chronic renal fail- tion. Although, these agents are effective, therapy is frequently ure [1, 2]. Decreased renal production of calcitriol (1,25 limited by hypercalcemia, hyperphosphatemia, and/or eleva- vitamin D ), hypocalcemia, and hyperphosphatemia are ϫ 3 tions in the calcium-phosphorus (Ca P) product. In clinical the major contributing factors to the development of studies, paricalcitol was shown to be effective at reducing PTH concentrations without causing significant hypercalcemia or secondary hyperparathyroidism [1, 3–5]. Calcitriol is syn- hyperphosphatemia as compared to placebo. A comparative thesized from previtamin D3 by hydroxylation on carbons study was undertaken in order to determine whether paricalci- 25 and 1 in the liver and kidney, respectively [6]. The tol provides a therapeutic advantage to calcitriol. -

A Randomised Clinical Study of Alfacalcidol and Paricalcitol
PHD THESIS DANISH MEDICAL JOURNAL A Randomised Clinical Study of Alfacalcidol and Paricalcitol Two vitamin D analogs for treatment of secondary hyperparathyroidism in chronic hemodialy- sis patients Ditte Hansen, MD stage 3, and are present in most patients when reaching dialysis. Ninety-six % of the hemodialysis patients (n = 76) in our depart- ment were at the time of screening for participants to the present This review has been accepted as a thesis together with three previously published papers by University of Copnehagen 20th of August 2011 and defended on 8th of study, treated for disturbances in the mineral metabolism. These October 2011 disturbances are associated with alterations in bone morphology, termed renal osteodystrophy and increased risk of skeletal frac- Tutors: Knud Rasmussen and Lisbet Brandi ture. The disturbances in the mineral metabolism are also associ- Official opponents: Klaus Ølgaard, Jens Dam Jensen and Tobias Larsson ated with vascular and other soft tissue calcification, and in turn increased cardiovascular morbidity and mortality. The systemic Correspondence: Department,of Medicine, Roskilde Hospital, Koegevej 7-13, 4000 disorder consisting of mineral disturbances, bone abnormalities Roskilde, Denmark and extraskeletal calcification, is defined as Chronic Kidney Dis- E-mail: [email protected] ease-Mineral and Bone Disorder (CKD-MBD).2 Secondary hyperparathyroidism and renal osteodystrophy Dan Med J 2012;59(2): B4400 When CKD develops 1,25-dihydroxyvitamin D levels decrease.3 This is partly due to decreased availability of the precursor 25- hydroxyvitamin D. The most important reason is the decreased THE THREE ORIGINAL PAPERS ARE 1α-hydroxylation of 25-hydroxyvitamin D in the kidney. -

Strategies for the Synthesis of 19-Nor-Vitamin D Analogs
pharmaceuticals Review Strategies for the Synthesis of 19-nor-Vitamin D Analogs Susana Fernández * and Miguel Ferrero * Departamento de Química Orgánica e Inorgánica, Universidad de Oviedo, 33006 Oviedo, Asturias, Spain * Correspondence: [email protected] (S.F.); [email protected] (M.F.); Tel.: +34-985-102-984 (S.F.); +34-985-105-013 (M.F.) Received: 18 June 2020; Accepted: 21 July 2020; Published: 22 July 2020 Abstract: 1α,25-Dihydroxyvitamin D3 [1α,25-(OH)2-D3], the hormonally active form of vitamin D3, classically regulates bone formation, calcium, and phosphate homeostasis. In addition, this hormone also exerts non-classical effects in a wide variety of target tissues and cell types, such as inhibition of the proliferation and stimulation of the differentiation of normal and malignant cells. However, to produce these actions, supraphysiological doses are required resulting in calcemic effects that limit the use of this natural hormone. During the past 30 years, many structurally modified analogs of the 1α,25-(OH)2-D3 have been synthesized in order to find derivatives that can dissociate the beneficial antiproliferative effects from undesired calcemic effects. Among these candidates, 1α,25-(OH)2-19-nor-D3 analogs have shown promise as good derivatives since they show equal or better activity relative to the parent hormone but with reduced calcemic effects. In this review, we describe the synthetic strategies to obtain the 19-nor-D3 derivatives and briefly describe their physiological activities. Keywords: vitamin D; 19-nor-vitamin D3; 19-nor-vitamin D2; synthesis; modified analogs 1. Introduction The steroid hormone 1α,25-dihydroxyvitamin D3 [1α,25-(OH)2-D3](2, Figure1) is the active form of vitamin D3 (1), which can be synthesized in the skin or obtained from dietary sources [1]. -

Policing Cancer: Vitamin D Arrests the Cell Cycle
International Journal of Molecular Sciences Review Policing Cancer: Vitamin D Arrests the Cell Cycle Sachin Bhoora 1 and Rivak Punchoo 1,2,* 1 Department of Chemical Pathology, Faculty of Health Sciences, University of Pretoria, Pretoria 0083, Gauteng, South Africa; [email protected] 2 National Health Laboratory Service, Tshwane Academic Division, Pretoria 0083, South Africa * Correspondence: [email protected] Received: 30 October 2020; Accepted: 26 November 2020; Published: 6 December 2020 Abstract: Vitamin D is a steroid hormone crucial for bone mineral metabolism. In addition, vitamin D has pleiotropic actions in the body, including anti-cancer actions. These anti-cancer properties observed within in vitro studies frequently report the reduction of cell proliferation by interruption of the cell cycle by the direct alteration of cell cycle regulators which induce cell cycle arrest. The most recurrent reported mode of cell cycle arrest by vitamin D is at the G1/G0 phase of the cell cycle. This arrest is mediated by p21 and p27 upregulation, which results in suppression of cyclin D and E activity which leads to G1/G0 arrest. In addition, vitamin D treatments within in vitro cell lines have observed a reduced C-MYC expression and increased retinoblastoma protein levels that also result in G1/G0 arrest. In contrast, G2/M arrest is reported rarely within in vitro studies, and the mechanisms of this arrest are poorly described. Although the relationship of epigenetics on vitamin D metabolism is acknowledged, studies exploring a direct relationship to cell cycle perturbation is limited. In this review, we examine in vitro evidence of vitamin D and vitamin D metabolites directly influencing cell cycle regulators and inducing cell cycle arrest in cancer cell lines. -

Oral Vitamin D Therapy in Patients with Psoriasis
nutrients Review Oral Vitamin D Therapy in Patients with Psoriasis Ana Maria Alexandra Stanescu 1 , Anca Angela Simionescu 2,* and Camelia Cristina Diaconu 3 1 Department of Family Medicine, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] 2 Department of Obstetrics and Gynecology, Filantropia Clinical Hospital, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania 3 Department of Internal Medicine, Clinical Emergency Hospital of Bucharest, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-213-188-930 Abstract: Vitamin D treatment is effective when applied topically to the skin for plaque-type psoriasis. Oral vitamin D supplementation might be effective as an adjuvant treatment option in psoriasis. This umbrella review aimed to highlight the current knowledge regarding the use of oral vitamin D for treatment of patients with psoriasis. We performed a literature search and identified 107 eligible full-text articles that were relevant to the research interest. Among these, 10 review articles were selected, and data were extracted. A data synthesis showed that only a few studies monitored oral vitamin D efficacy in patients with psoriasis. No studies investigated the optimal dose of systemic vitamin D in psoriasis. However, most studies did not observe side effects for doses within a relatively narrow range (0.25 to 2 µg/day). These results suggest that more large-scale studies are needed to determine the efficacy, optimal dose, and adverse effects of vitamin D administration in patients with psoriasis. Keywords: psoriasis; oral vitamin D; treatment 1. -

The Vitamin D System in Humans and Mice: Similar but Not the Same
Review The Vitamin D System in Humans and Mice: Similar but Not the Same Ewa Marcinkowska Department of Biotechnology, University of Wroclaw, Joliot-Curie 14a, 50-383 Wroclaw, Poland; [email protected]; Tel.: +48-71-375-2929 Received: 7 November 2019; Accepted: 7 January 2020; Published: 10 January 2020 Abstract: Vitamin D is synthesized in the skin from 7-dehydrocholesterol subsequently to exposure to UVB radiation or is absorbed from the diet. Vitamin D undergoes enzymatic conversion to its active form, 1,25-dihydroxyvitamin D (1,25D), a ligand to the nuclear vitamin D receptor (VDR), which activates target gene expression. The best-known role of 1,25D is to maintain healthy bones by increasing the intestinal absorption and renal reuptake of calcium. Besides bone maintenance, 1,25D has many other functions, such as the inhibition of cell proliferation, induction of cell differentiation, augmentation of innate immune functions, and reduction of inflammation. Significant amounts of data regarding the role of vitamin D, its metabolism and VDR have been provided by research performed using mice. Despite the fact that humans and mice share many similarities in their genomes, anatomy and physiology, there are also differences between these species. In particular, there are differences in composition and regulation of the VDR gene and its expression, which is discussed in this article. Keywords: vitamin D; vitamin D receptor; human; murine; drug testing 1. Introduction Different animal models have been used in biomedical research. Simple organisms, such as yeast, fruit flies, C. elegans and zebrafish are useful to study function of particular genes and their roles in development. -

Effectiveness of Vitamin D Analogues in Treating Large Tumors and During Prolonged Use in Murine Retinoblastoma Models
LABORATORY SCIENCES Effectiveness of Vitamin D Analogues in Treating Large Tumors and During Prolonged Use in Murine Retinoblastoma Models Daniel M. Albert, MD, MS; Amit Kumar, MD; Stephen A. Strugnell, PhD; Soesiawati R. Darjatmoko, MS; Janice M. Lokken; Mary J. Lindstrom, PhD; Sarit Patel, MD Objective: To investigate the effectiveness of the vita- smaller tumor size compared with its control (PϽ.001). min D analogues 1,25-(OH)2-16-ene-23-yne vitamin D3 No significant difference was seen between the 16,23-D3 ␣ ␣ (16,23-D3) and 1 -hydroxyvitamin D2 (1 -OH-D2)inin- group and its control. Some toxic effects related to hy- hibiting retinoblastoma growth in large tumors in a xe- percalcemia were seen in both studies. nograft model and with prolonged use in a transgenic model. Conclusions: In athymic mice in the large-tumor study, ␣ both 1 -OH-D2 and 16,23-D3 were effective in inhibit- Methods: For the large-tumor study, the xenograft athy- ing tumor growth compared with controls. In the long- ␣ mic mouse/human retinoblastoma cell (Y-79) model was term study, 1 -OH-D2 inhibited tumor growth but used. Subcutaneous tumors were allowed to grow to an 16,23-D3 did not. Effective doses of both compounds average volume of 1600 mm3. Systemic treatment with caused hypercalcemia and a significant increase in mor- 1 of the vitamin D analogues or with vehicle (control tality. groups) was carried out for 5 weeks. For the long-term  ␣ study, transgenic –luteinizing hormone–large T anti- Clinical Relevance: Use of 1 -OH-D2 inhibited tu- gen (LH-Tag) mice were systemically treated with 1 of mor growth in large tumors and with long-term treat- the 2 compounds or vehicle (control groups) for up to ment compared with controls. -
![DBOOST® Capsules/Sachet [Vitamin D3 (Cholecalciferol)]](https://docslib.b-cdn.net/cover/8883/dboost%C2%AE-capsules-sachet-vitamin-d3-cholecalciferol-3798883.webp)
DBOOST® Capsules/Sachet [Vitamin D3 (Cholecalciferol)]
DBOOST® Capsules/Sachet [Vitamin D3 (cholecalciferol)] COMPOSITION Each Capsule/Sachet contains: Cholecalciferol 60,000 IU PHARMACOLOGY Vitamin D is a fat-soluble vitamin and has properties of both vitamins and minerals. Vitamin D is essential for the absorption and utilization of calcium and phosphate and aids in the mobilization of bone calcium and maintenance of serum calcium concentrations. Cholecalciferol (Vitamin D3) is synthesized in the skin on exposure to ultraviolet radiation. Cholecalciferol is also present in fish liver oils. Ergocalciferol (Vitamin D2) is produced by ultraviolet irradiation of a provitamin D sterol (ergosterol) which occurs in yeast and fungi. Both of these agents who have equal biologic activity are metabolized in the liver to calcifediol (25 hydroxycholecalciferol) which is then hydroxylated in the kidney to calcitriol (1, 25 dihydroxycholecalciferol). Calcitriol is considered the most active form. Dihydrotachysterol is produced by synthetic reduction of ergocalciferol. Patients with chronic renal disease cannot convert calcifediol to calcitriol. Alfacalcidol (1 α hydroxyvitamin D3), a synthetic analogue of calcitriol, is rapidly converted in the liver to calcitriol, bypassing the renal conversion step. Because alfacalcidol, calcitriol and dihydrotachysterol do not require renal hydroxylation, they are useful in patients with renal failure. INDICATIONS Vitamin D analogues are used in treatment of refractory rickets (Vitamin D- resistant rickets), familial hypophosphatemia and hypoparathyroidism, and in the management of hypocalcemia and renal osteodystrophy in patients with chronic renal failure undergoing dialysis. Vitamin D is used in conjunction with calcium in the management and prevention of primary or corticosteroid-induced osteoporosis. Vitamin D supplementation is indicated when dietary intake is insufficient, e.g., breast-fed infants. -

Effects of Vitamin D3 and Its Chemical Analogs on the Growth of Hodgkin's
City University of New York (CUNY) CUNY Academic Works Publications and Research Bronx Community College 2019 Effects of vitamin D3 and its chemical analogs on the growth of Hodgkin’s lymphoma, in vitro Rajendra Ghardbaran CUNY Bronx Community College Bo Zhang CUNY Lehman College Luis Valerio CUNY Bronx Community College Onyekwere Onwumere CUNY Lehman College Madeline Wong CUNY Lehman College See next page for additional authors How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/bx_pubs/67 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Authors Rajendra Ghardbaran, Bo Zhang, Luis Valerio, Onyekwere Onwumere, Madeline Wong, Jason Mighty, and Stephen Redenti This article is available at CUNY Academic Works: https://academicworks.cuny.edu/bx_pubs/67 Gharbaran et al. BMC Res Notes (2019) 12:216 https://doi.org/10.1186/s13104-019-4241-0 BMC Research Notes RESEARCH NOTE Open Access Efects of vitamin D3 and its chemical analogs on the growth of Hodgkin’s lymphoma, in vitro Rajendra Gharbaran1,2*, Bo Zhang2, Luis Valerio1,2, Onyekwere Onwumere2,3, Madeline Wong2, Jason Mighty2,3 and Stephen Redenti2,3 Abstract Objective: Vitamin D receptor (VDR) activities have been noted for a number of B cell malignancies which showed varying sensitivities to vitamin D3 (1,25-dihydroxyvitamin D3, VD3, calcitriol) and its synthetic analogs. The objective of this study was to address the potential efects of VD3 and vitamin D3 analogs (VDAs) on the growth of Hodgkin’s lymphoma (HL), a malignant pathology of B cell origin, in vitro.