Cholecalciferol) Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Anthem Blue Cross Drug Formulary
Erythromycin/Sulfisoxazole (generic) INTRODUCTION Penicillins ...................................................................... Anthem Blue Cross uses a formulary Amoxicillin (generic) (preferred list of drugs) to help your doctor Amoxicillin/Clavulanate (generic/Augmentin make prescribing decisions. This list of drugs chew/XR) is updated quarterly, by a committee Ampicillin (generic) consisting of doctors and pharmacists, so that Dicloxacillin (generic) the list includes drugs that are safe and Penicillin (generic) effective in the treatment of diseases. If you Quinolones ..................................................................... have any questions about the accessibility of Ciprofloxacin/XR (generic) your medication, please call the phone number Levofloxacin (Levaquin) listed on the back of your Anthem Blue Cross Sulfonamides ................................................................ member identification card. Erythromycin/Sulfisoxazole (generic) In most cases, if your physician has Sulfamethoxazole/Trimethoprim (generic) determined that it is medically necessary for Sulfisoxazole (generic) you to receive a brand name drug or a drug Tetracyclines .................................................................. that is not on our list, your physician may Doxycycline hyclate (generic) indicate “Dispense as Written” or “Do Not Minocycline (generic) Substitute” on your prescription to ensure Tetracycline (generic) access to the medication through our network ANTIFUNGAL AGENTS (ORAL) _________________ of community -

Drug Consumption in 2017 - 2020
Page 1 Drug consumption in 2017 - 2020 2020 2019 2018 2017 DDD/ DDD/ DDD/ DDD/ 1000 inhab./ Hospital 1000 inhab./ Hospital 1000 inhab./ Hospital 1000 inhab./ Hospital ATC code Subgroup or chemical substance day % day % day % day % A ALIMENTARY TRACT AND METABOLISM 322,79 3 312,53 4 303,08 4 298,95 4 A01 STOMATOLOGICAL PREPARATIONS 14,28 4 12,82 4 10,77 6 10,46 7 A01A STOMATOLOGICAL PREPARATIONS 14,28 4 12,82 4 10,77 6 10,46 7 A01AA Caries prophylactic agents 11,90 3 10,48 4 8,42 5 8,45 7 A01AA01 sodium fluoride 11,90 3 10,48 4 8,42 5 8,45 7 A01AA03 olaflur 0,00 - 0,00 - 0,00 - 0,00 - A01AB Antiinfectives for local oral treatment 2,36 8 2,31 7 2,31 7 2,02 7 A01AB03 chlorhexidine 2,02 6 2,10 7 2,09 7 1,78 7 A01AB11 various 0,33 21 0,21 0 0,22 0 0,24 0 A01AD Other agents for local oral treatment 0,02 0 0,03 0 0,04 0 - - A01AD02 benzydamine 0,02 0 0,03 0 0,04 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 73,05 3 71,13 3 69,32 3 68,35 3 A02A ANTACIDS 2,23 1 2,22 1 2,20 1 2,30 1 A02AA Magnesium compounds 0,07 22 0,07 22 0,08 22 0,10 19 A02AA04 magnesium hydroxide 0,07 22 0,07 22 0,08 22 0,10 19 A02AD Combinations and complexes of aluminium, 2,17 0 2,15 0 2,12 0 2,20 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 2,17 0 2,15 0 2,12 0 2,20 0 A02B DRUGS FOR PEPTIC ULCER AND 70,82 3 68,91 3 67,12 3 66,05 4 GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 0,17 7 0,74 4 1,10 4 1,11 5 A02BA02 ranitidine 0,00 1 0,63 3 0,99 3 0,99 4 A02BA03 famotidine 0,16 7 0,11 8 0,11 10 0,12 9 A02BB Prostaglandins 0,04 62 -

A New Role for Vitamin D Receptor Activation in Chronic Kidney Disease
A NEW ROLE FOR VITAMIN D RECEPTOR ACTIVATION IN CHRONIC KIDNEY DISEASE José M Valdivielso 1, Jorge Cannata-Andía2, Blai Coll 1 and Elvira Fernández 1 1Laboratorio de Nefrología Experimental, Hospital Universitario Arnau de Vilanova, IRBLLEIDA, REDinREN ISCIII Lleida, Spain. 2Servicio de Metabolismo Óseo y Mineral. Hospital Universitario Central de Asturias. Instituto “Reina Sofía” de Investigación. REDinREN ISCIII. Universidad de Oviedo Running Title: New role of VDRA Correspondence to: Dr. José M Valdivielso, Laboratorio de Nefrología Experimental, IRBLLEIDA Hospital Universitari Arnau de Vilanova Rovira Roure 80 25198 Lleida, Spain. Phone: +34 973003650 Fax: +34 973702213 e-mail: [email protected] Abstract Vitamin D has proven to be much more than a simple ‘calcium hormone’. The fact that the vitamin D receptor has been found in cells not related to mineral metabolism supports that statement. The interest of nephrologists in Vitamin D and its effects beyond mineral metabolism has increased over the last few years, evidencing the importance of this so-called ‘sunshine hormone’. In the present review, we highlight the most recent developments in the traditional use of vitamin D in chronic kidney disease (CKD) patients, namely the control of secondary hyperparathyroidism (sHPT). Furthermore, we also explore the data available regarding the new possible therapeutic uses of vitamin D for the treatment of other complications present in CKD patients, such as vascular calcification, left ventricular hypertrophy or proteinuria. Finally, some still scarce but very promising data regarding a possible role of vitamin D in kidney transplant patients are also reviewed. The available data point to a potential beneficial effect of vitamin D in CKD patients beyond the control of mineral metabolism. -
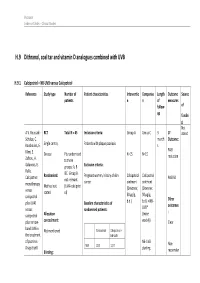
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

(12) United States Patent (10) Patent No.: US 6,187,331 B1 Itoh Et Al
USOO6187331B1 (12) United States Patent (10) Patent No.: US 6,187,331 B1 Itoh et al. (45) Date of Patent: Feb. 13, 2001 (54) COMPOSITION FOR PROPHYLAXIS AND/ 5-508655 12/1993 (JP). OR TREATMENT OF DRY SYNDROME WO 96/29079 9/1996 (WO). COMPRISING WITAMIN ID WO 97/18817 5/1997 (WO). WO 98/51313 11/1998 (WO). (75) Inventors: Seiji Itoh, Mobara; Yasuo Ishii, OTHER PUBLICATIONS Kawaguchi; Katsuhiko Mukai, Kashiwa; Kiyoshi Kita, Tokyo, all of Leslie Bendra Sabbagh, “Sunlight May be Enemy of Heal (JP) ing Excimer PRK', Ocular Surgery News, vol. 9, No. 11, pp. 21–22, Jun. 1, 1991. (73) Assignee: New Vision Co., Ltd., Tokyo (JP) The Vitamins, pp. 54–55, 104-115, 150-177, 206–221, Academic Press, Inc.,1992. (*) Notice: Under 35 U.S.C. 154(b), the term of this Dryeye Brochure, pp. 10-13, Nippon Hyoronsha, Japan, patent shall be extended for 0 days. 1992. Paul Riordan-Eva, “Preventive Ophthalmology”, Appleton (21) Appl. No.: 09/575,987 & Lange, pp. 388-395, 1993. Ganka New Insight 5, pp. 132-143, Medical View-sha, (22) Filed: May 23, 2000 Japan, 1995. (Under 37 CFR 1.47) Olive Sheets et al., “The Effect of Ultra-Violet Rays on Rats, Deprived of Vitamine A in Their Diet”, Scientific Proceed Related U.S. Application Data ings, vol. 20, pp. 80-81, 1992. G. F. Powers et al., “The Influence of Light and Darkness (63) Continuation of application No. 08/872,052, filed on Jun. Upon the Development of exerophthalmia in the Rat', 10, 1997. Scientific Proceedings, vol. -

Estonian Statistics on Medicines 2006–2010
Ravimiamet State Agency of Medicines Eesti ravimistatistika 2006–2010 Estonian Statistics on Medicines 2006–2010 1 Toimetanud/ Edited by: Aivi Themas, Ott Laius Autoriõigus/ Copyright: Ravimiamet, 2011 Väljaande andmete kasutamisel või tsiteerimisel palume viidata allikale. When using or quoting the data included in this issue, please indicate the source. Kirjastanud/ Published by: Ravimiamet State Agency of Medicines Nooruse 1, 50411 Tartu Telefon 737 4140 Faks 737 4142 E-post [email protected] Trükkinud/Printed by: OÜ Paar ISSN 1736-5813 2 Eessõna Foreword Käesolev raamat on statistiline kokkuvõte This book is a statistical summary of the ravimikasutamise andmetest Eestis aastatel Estonian drug consumption data in 2006 – 2006–2010. Tulemused põhinevad ravimite 2010. The figures included in the book rep- hulgimüüjate esitatud aruannetel, mis kajas- resent sales from the wholesalers to gene- tavad ravimite müüki üld- ja haiglaapteeki- ral and hospital pharmacies and to other dele ning teistele asutustele (riigi- ja teadus- institutions (state- and scientific institu- asutused). tions). Eesti ravimikasutamise andmete võrrelda- In order to provide better possibilities for shar- vuse tagamiseks teiste riikidega on tulemu- ing experiences and making international sed esitatud anatoomilis-terapeutilis-keemi- comparisons, the Anatomical-Therapeutic- lise (ATC) klassifikatsiooni alusel, definee- Chemical (ATC) classification of medicines ritud päevadooside arvuna tuhande inimese and the Defined Daily Dose (DDD) methodo- kohta ööpäevas (DPD/1000/ ööpäevas). logy recommended by the World Health Defineeritud päevadoos (DPD) on kokku- Organization is used. The DDD is the leppeline suurus, mis on Maailma Tervise- assumed average dose per day for the drug organisatsiooni (WHO) poolt välja töötatud used in its main indication in adults. -

Paricalcitol Versus Calcitriol in the Treatment of Secondary Hyperparathyroidism
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 63 (2003), pp. 1483–1490 Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism STUART M. SPRAGUE,FRANCISCO LLACH,MICHAEL AMDAHL,CAROL TACCETTA, and DANIEL BATLLE Division of Nephrology/Hypertension and Department of Medicine, Northwestern University Feinberg School of Medicine, Evanston and Chicago, Illinois; Georgetown University Medical School, Washington, D.C.; and Abbott Laboratories, North Chicago, Illinois Paricalcitol versus calcitriol in the treatment of secondary hyper- Osteitis fibrosa cystica, resulting from poorly con- parathyroidism. trolled secondary hyperparathyroidism, remains a com- Background. Management of secondary hyperparathyroid- mon manifestation of renal osteodystrophy and causes ism has included the use of active vitamin D or vitamin D ana- logs for the suppression of parathyroid hormone (PTH) secre- significant morbidity in patients with chronic renal fail- tion. Although, these agents are effective, therapy is frequently ure [1, 2]. Decreased renal production of calcitriol (1,25 limited by hypercalcemia, hyperphosphatemia, and/or eleva- vitamin D ), hypocalcemia, and hyperphosphatemia are ϫ 3 tions in the calcium-phosphorus (Ca P) product. In clinical the major contributing factors to the development of studies, paricalcitol was shown to be effective at reducing PTH concentrations without causing significant hypercalcemia or secondary hyperparathyroidism [1, 3–5]. Calcitriol is syn- hyperphosphatemia as compared to placebo. A comparative thesized from previtamin D3 by hydroxylation on carbons study was undertaken in order to determine whether paricalci- 25 and 1 in the liver and kidney, respectively [6]. The tol provides a therapeutic advantage to calcitriol. -

A Randomised Clinical Study of Alfacalcidol and Paricalcitol
PHD THESIS DANISH MEDICAL JOURNAL A Randomised Clinical Study of Alfacalcidol and Paricalcitol Two vitamin D analogs for treatment of secondary hyperparathyroidism in chronic hemodialy- sis patients Ditte Hansen, MD stage 3, and are present in most patients when reaching dialysis. Ninety-six % of the hemodialysis patients (n = 76) in our depart- ment were at the time of screening for participants to the present This review has been accepted as a thesis together with three previously published papers by University of Copnehagen 20th of August 2011 and defended on 8th of study, treated for disturbances in the mineral metabolism. These October 2011 disturbances are associated with alterations in bone morphology, termed renal osteodystrophy and increased risk of skeletal frac- Tutors: Knud Rasmussen and Lisbet Brandi ture. The disturbances in the mineral metabolism are also associ- Official opponents: Klaus Ølgaard, Jens Dam Jensen and Tobias Larsson ated with vascular and other soft tissue calcification, and in turn increased cardiovascular morbidity and mortality. The systemic Correspondence: Department,of Medicine, Roskilde Hospital, Koegevej 7-13, 4000 disorder consisting of mineral disturbances, bone abnormalities Roskilde, Denmark and extraskeletal calcification, is defined as Chronic Kidney Dis- E-mail: [email protected] ease-Mineral and Bone Disorder (CKD-MBD).2 Secondary hyperparathyroidism and renal osteodystrophy Dan Med J 2012;59(2): B4400 When CKD develops 1,25-dihydroxyvitamin D levels decrease.3 This is partly due to decreased availability of the precursor 25- hydroxyvitamin D. The most important reason is the decreased THE THREE ORIGINAL PAPERS ARE 1α-hydroxylation of 25-hydroxyvitamin D in the kidney. -

Strategies for the Synthesis of 19-Nor-Vitamin D Analogs
pharmaceuticals Review Strategies for the Synthesis of 19-nor-Vitamin D Analogs Susana Fernández * and Miguel Ferrero * Departamento de Química Orgánica e Inorgánica, Universidad de Oviedo, 33006 Oviedo, Asturias, Spain * Correspondence: [email protected] (S.F.); [email protected] (M.F.); Tel.: +34-985-102-984 (S.F.); +34-985-105-013 (M.F.) Received: 18 June 2020; Accepted: 21 July 2020; Published: 22 July 2020 Abstract: 1α,25-Dihydroxyvitamin D3 [1α,25-(OH)2-D3], the hormonally active form of vitamin D3, classically regulates bone formation, calcium, and phosphate homeostasis. In addition, this hormone also exerts non-classical effects in a wide variety of target tissues and cell types, such as inhibition of the proliferation and stimulation of the differentiation of normal and malignant cells. However, to produce these actions, supraphysiological doses are required resulting in calcemic effects that limit the use of this natural hormone. During the past 30 years, many structurally modified analogs of the 1α,25-(OH)2-D3 have been synthesized in order to find derivatives that can dissociate the beneficial antiproliferative effects from undesired calcemic effects. Among these candidates, 1α,25-(OH)2-19-nor-D3 analogs have shown promise as good derivatives since they show equal or better activity relative to the parent hormone but with reduced calcemic effects. In this review, we describe the synthetic strategies to obtain the 19-nor-D3 derivatives and briefly describe their physiological activities. Keywords: vitamin D; 19-nor-vitamin D3; 19-nor-vitamin D2; synthesis; modified analogs 1. Introduction The steroid hormone 1α,25-dihydroxyvitamin D3 [1α,25-(OH)2-D3](2, Figure1) is the active form of vitamin D3 (1), which can be synthesized in the skin or obtained from dietary sources [1]. -

Pharmaceutical Composition and Dosage Forms for Administration of Hydrophobic Drugs
(19) & (11) EP 2 246 049 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 03.11.2010 Bulletin 2010/44 A61K 31/355 (2006.01) A61K 31/56 (2006.01) C07J 53/00 (2006.01) (21) Application number: 10173114.9 (22) Date of filing: 24.05.2004 (84) Designated Contracting States: • Fikstad, David T. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Salt Lake City, UT 84102 (US) HU IE IT LI LU MC NL PL PT RO SE SI SK TR • Zhang, Huiping Salt Lake City, UT 844121 (US) (30) Priority: 22.05.2003 US 444935 • Giliyar, Chandrashekar Salt Lake City, UT 84102 (US) (62) Document number(s) of the earlier application(s) in accordance with Art. 76 EPC: (74) Representative: Walker, Ross Thomson 04753162.9 / 1 624 855 Potts, Kerr & Co. 15 Hamilton Square (71) Applicant: Lipocine, Inc. Birkenhead Salt Lake City, UT 84103 (US) Merseyside CH41 6BR (GB) (72) Inventors: Remarks: • Chen, Feng-Jing This application was filed on 17-08-2010 as a Salt Lake City, UT 84111 (US) divisional application to the application mentioned • Patel, Mahesh V. under INID code 62. Salt Lake City, UT 84124 (US) (54) Pharmaceutical composition and dosage forms for administration of hydrophobic drugs (57) Pharmaceutical compositions and dosage tion with improved dispersion of both the active agent forms for administration of hydrophobic drugs, particu- and the solubilizer. As a result of the improved dispersion, larly steroids, are provided. The pharmaceutical compo- the pharmaceutical composition has improved bioavail- sitions include a therapeutically effective amount of a hy- ability upon administration. -

Drug Consumption at Wholesale Prices in 2017 - 2020
Page 1 Drug consumption at wholesale prices in 2017 - 2020 2020 2019 2018 2017 Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. ATC code Subgroup or chemical substance price/1000 € % price/1000 € % price/1000 € % price/1000 € % A ALIMENTARY TRACT AND METABOLISM 321 590 7 309 580 7 300 278 7 295 060 8 A01 STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01A STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01AA Caries prophylactic agents 663 8 611 11 619 12 1 042 11 A01AA01 sodium fluoride 610 8 557 12 498 15 787 14 A01AA03 olaflur 53 1 54 1 50 1 48 1 A01AA51 sodium fluoride, combinations - - - - 71 1 206 1 A01AB Antiinfectives for local oral treatment 1 266 10 1 101 6 1 052 6 944 6 A01AB03 chlorhexidine 930 6 885 7 825 7 706 7 A01AB11 various 335 21 216 0 227 0 238 0 A01AB22 doxycycline - - 0 100 0 100 - - A01AC Corticosteroids for local oral treatment 113 1 153 1 135 1 143 1 A01AC01 triamcinolone 113 1 153 1 135 1 143 1 A01AD Other agents for local oral treatment 49 0 72 0 104 0 - - A01AD02 benzydamine 49 0 72 0 104 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 30 885 4 32 677 4 35 102 5 37 644 7 A02A ANTACIDS 3 681 1 3 565 1 3 357 1 3 385 1 A02AA Magnesium compounds 141 22 151 22 172 22 155 19 A02AA04 magnesium hydroxide 141 22 151 22 172 22 155 19 A02AD Combinations and complexes of aluminium, 3 539 0 3 414 0 3 185 0 3 231 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 3 539 0 3 414 0 3 185 0 3 231 0 A02B DRUGS FOR PEPTIC ULCER AND 27 205 5 29 112 4 31 746 5 34 258 8