DBOOST® Capsules/Sachet [Vitamin D3 (Cholecalciferol)]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Extended-Release 30 Mcg Capsules Service Request Form Fax: 1-844-660-7083 | Phone: 1-844-414-Opko (6756) E-Mail: [email protected]
RAYALDEE® (CALCIFEDIOL) EXTENDED-RELEASE 30 MCG CAPSULES SERVICE REQUEST FORM FAX: 1-844-660-7083 | PHONE: 1-844-414-OPKO (6756) E-MAIL: [email protected] 1. Patient Information Please complete all fields to prevent any delays. New start to Rayaldee® therapy Existing patient on therapy E-mail Address: Preferred Method of Contact: Cell Phone Home Phone Email Text First Name Last Name SS # (Last 4 only) Preferred Time of Contact: Morning Afternoon Evening Male Female Ok to leave a message: Yes No Date of Birth (MM/DD/YYYY) Primary Language: English Spanish Other:________________________________ 2. Patient Insurance Information Address Attached is a copy of both sides of the patient's insurance card City State ZIP Primary Insurance Phone # Cell Phone Home Phone Policy Holder Name Relationship to Patient Alternate Contact or Healthcare Proxy First Name, Last Name and Phone Insurance ID # Group # 3. Patient Clinical Information Please include supporting clinical documentation. ● ICD-10 Code: ● Lab Values & dates: Please check box 1. or 2. below 25(OH)D__________|__________ Calcium__________|__________ (N18.3) CKD stage 3, (N25.81) Secondary hyperparathyroidism, and 1. value date value date (E55.9) Vitamin D deficiency 2. (N18.4) CKD stage 4, (N25.81) Secondary hyperparathyroidism, and (E55.9) Vitamin D deficiency ● Therapies within the previous 6 months: No previous therapies Hectorol® (doxercalciferol) Rocaltrol® (calcitriol) OTC Vitamin D2 ® OTC Vitamin D3 Prescription Vitamin D2 (ergocalciferol) Zemplar (paricalcitol) 4. Prescriber Information Specialty of Prescriber: Nephrologist PCP Endocrinologist Internist First Name Last Name Phone Fax Practice Name Oce Contact Preferred Time of Contact Address NPI # City State ZIP 5. -

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Daily Lifestyle Modifications to Improve Quality of Life And
brain sciences Review Daily Lifestyle Modifications to Improve Quality of Life and Survival in Glioblastoma: A Review Sarah Travers and N. Scott Litofsky * Division of Neurosurgery, University of Missouri School of Medicine, Columbia, MO 65212, USA; [email protected] * Correspondence: [email protected] Abstract: Survival in glioblastoma remains poor despite advancements in standard-of-care treatment. Some patients wish to take a more active role in their cancer treatment by adopting daily lifestyle changes to improve their quality of life or overall survival. We review the available literature through PubMed and Google Scholar to identify laboratory animal studies, human studies, and ongoing clinical trials. We discuss which health habits patients adopt and which have the most promise in glioblastoma. While results of clinical trials available on these topics are limited, dietary restrictions, exercise, use of supplements and cannabis, and smoking cessation all show some benefit in the comprehensive treatment of glioblastoma. Marital status also has an impact on survival. Further clinical trials combining standard treatments with lifestyle modifications are necessary to quantify their survival advantages. Keywords: survival in glioblastoma; dietary restriction in glioblastoma; cannabis use in glioblastoma; supplementation in glioblastoma; glioblastoma health modifications Citation: Travers, S.; Litofsky, N.S. Daily Lifestyle Modifications to 1. Introduction Improve Quality of Life and Survival Glioblastoma remains the most aggressive and deadly form of primary brain tumor, in Glioblastoma: A Review. Brain Sci. with average survival rates ranging from 7.8 to 23.4 months after diagnosis [1]. Maximal 2021, 11, 533. https://doi.org/ surgical resection followed by radiation and temozolomide have become standard of 10.3390/brainsci11050533 care [2,3]. -

Acne Vulgaris and Intake of Selected Dietary Nutrients—A Summary of Information
healthcare Review Acne Vulgaris and Intake of Selected Dietary Nutrients—A Summary of Information Aleksandra Podgórska †, Anna Pu´scion-Jakubik*,† , Renata Markiewicz-Zukowska˙ , Krystyna Joanna Gromkowska-K˛epkaand Katarzyna Socha Department of Bromatology, Faculty of Pharmacy with the Division of Laboratory Medicine, Medical University of Białystok, Mickiewicza 2D Street, 15-222 Białystok, Poland; [email protected] (A.P.); [email protected] (R.M.-Z.);˙ [email protected] (K.J.G.-K.); [email protected] (K.S.) * Correspondence: [email protected]; Tel.: +48-8574-854-69 † Contributed equally. Abstract: Acne vulgaris (AV) is a chronic disease that affects a significant percentage of the world’s population. Its development is influenced by both external and internal factors. The purpose of this review is to demonstrate the effect of basic nutrient intake on the exacerbation or alleviation of AV lesions. A retrospective review of publications in PubMed regarding diet therapy and the impact of individual nutrient intake on the skin condition of patients was conducted. Ingestion of products with a high glycaemic index may indirectly lead to sebum overproduction, which promotes infection with Cutibacterium acnes and causes inflammation. Consumption of certain dairy products may result Citation: Podgórska, A.; in skin deterioration caused by the presence of hormones in these products, i.e., progesterone and Pu´scion-Jakubik,A.; testosterone precursors. The beneficial effect of fatty acids on the skin is manifested by the reduction Markiewicz-Zukowska,˙ R.; Gromkowska-K˛epka,K.J.; Socha, K. in inflammation. Of significance in AV treatment are vitamins A, C, D, E and B, as well as mineral Acne Vulgaris and Intake of Selected elements zinc and selenium. -

Vitamin D and Its Analogues Decrease Amyloid- (A) Formation
International Journal of Molecular Sciences Article Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation Marcus O. W. Grimm 1,2,3,*,† ID , Andrea Thiel 1,† ID , Anna A. Lauer 1 ID , Jakob Winkler 1, Johannes Lehmann 1,4, Liesa Regner 1, Christopher Nelke 1, Daniel Janitschke 1,Céline Benoist 1, Olga Streidenberger 1, Hannah Stötzel 1, Kristina Endres 5, Christian Herr 6 ID , Christoph Beisswenger 6, Heike S. Grimm 1 ID , Robert Bals 6, Frank Lammert 4 and Tobias Hartmann 1,2,3 1 Experimental Neurology, Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany; [email protected] (A.T.); [email protected] (A.A.L.); [email protected] (J.W.); [email protected] (J.L.); [email protected] (L.R.); [email protected] (C.N.); [email protected] (D.J.); [email protected] (C.B.); [email protected] (O.S.); [email protected] (H.S.); [email protected] (H.S.G.); [email protected] (T.H.) 2 Neurodegeneration and Neurobiology, Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany 3 Deutsches Institut für DemenzPrävention (DIDP), Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany 4 Department of Internal Medicine II–Gastroenterology, Saarland University Hospital, Saarland University, Kirrberger Str. 100, 66421 Homburg/Saar, Germany; [email protected] 5 Department of Psychiatry and Psychotherapy, Clinical Research Group, University Medical Centre Johannes Gutenberg, University of Mainz, Untere Zahlbacher Str. 8, 55131 Mainz, Germany; [email protected] 6 Department of Internal Medicine V–Pulmonology, Allergology, Respiratory Intensive Care Medicine, Saarland University Hospital, Kirrberger Str. -

A Clinical Update on Vitamin D Deficiency and Secondary
References 1. Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic 17. Ennis JL, Worcester EM, Coe FL, Sprague SM. Current recommended 32. Thimachai P, Supasyndh O, Chaiprasert A, Satirapoj B. Efficacy of High 38. Kramer H, Berns JS, Choi MJ, et al. 25-Hydroxyvitamin D testing and kidney disease. Clin J Am Soc Nephrol. 2008;3:1144-1151. 25-hydroxyvitamin D targets for chronic kidney disease management vs. Conventional Ergocalciferol Dose for Increasing 25-Hydroxyvitamin supplementation in CKD: an NKF-KDOQI controversies report. Am J may be too low. J Nephrol. 2016;29:63-70. D and Suppressing Parathyroid Hormone Levels in Stage III-IV CKD Kidney Dis. 2014;64:499-509. 2. Hollick MF. Vitamin D: importance in the prevention of cancers, type 1 with Vitamin D Deficiency/Insufficiency: A Randomized Controlled Trial. diabetes, heart disease, and osteoporosis. Am J Clin Nutr 18. OPKO. OPKO diagnostics point-of-care system. Available at: http:// J Med Assoc Thai. 2015;98:643-648. 39. Jetter A, Egli A, Dawson-Hughes B, et al. Pharmacokinetics of oral 2004;79:362-371. www.opko.com/products/point-of-care-diagnostics/. Accessed vitamin D(3) and calcifediol. Bone. 2014;59:14-19. September 2 2015. 33. Kovesdy CP, Lu JL, Malakauskas SM, et al. Paricalcitol versus 3. Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 40. Petkovich M, Melnick J, White J, et al. Modified-release oral calcifediol of vitamin D status and cancer incidence and mortality in men. -

A New Role for Vitamin D Receptor Activation in Chronic Kidney Disease
A NEW ROLE FOR VITAMIN D RECEPTOR ACTIVATION IN CHRONIC KIDNEY DISEASE José M Valdivielso 1, Jorge Cannata-Andía2, Blai Coll 1 and Elvira Fernández 1 1Laboratorio de Nefrología Experimental, Hospital Universitario Arnau de Vilanova, IRBLLEIDA, REDinREN ISCIII Lleida, Spain. 2Servicio de Metabolismo Óseo y Mineral. Hospital Universitario Central de Asturias. Instituto “Reina Sofía” de Investigación. REDinREN ISCIII. Universidad de Oviedo Running Title: New role of VDRA Correspondence to: Dr. José M Valdivielso, Laboratorio de Nefrología Experimental, IRBLLEIDA Hospital Universitari Arnau de Vilanova Rovira Roure 80 25198 Lleida, Spain. Phone: +34 973003650 Fax: +34 973702213 e-mail: [email protected] Abstract Vitamin D has proven to be much more than a simple ‘calcium hormone’. The fact that the vitamin D receptor has been found in cells not related to mineral metabolism supports that statement. The interest of nephrologists in Vitamin D and its effects beyond mineral metabolism has increased over the last few years, evidencing the importance of this so-called ‘sunshine hormone’. In the present review, we highlight the most recent developments in the traditional use of vitamin D in chronic kidney disease (CKD) patients, namely the control of secondary hyperparathyroidism (sHPT). Furthermore, we also explore the data available regarding the new possible therapeutic uses of vitamin D for the treatment of other complications present in CKD patients, such as vascular calcification, left ventricular hypertrophy or proteinuria. Finally, some still scarce but very promising data regarding a possible role of vitamin D in kidney transplant patients are also reviewed. The available data point to a potential beneficial effect of vitamin D in CKD patients beyond the control of mineral metabolism. -

Toiminta Unita on Ulla La Mungukurti |
TOIMINTAUNITA USON 20180071390A1ULLA LA MUNGUKURTI | ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2018/ 0071390 A1 PATEL et al. (43 ) Pub . Date : Mar . 15 , 2018 ( 54 ) COMPOSITIONS OF PHARMACEUTICAL A61K 9 / 06 (2006 .01 ) ACTIVES CONTAINING DIETHYLENE A61K 9 /00 (2006 .01 ) GLYCOL MONOETHYL ETHER OR OTHER A61K 31 /573 ( 2006 .01 ) ALKYL DERIVATIVES A61K 31/ 565 ( 2006 .01 ) A61K 31/ 4439 ( 2006 . 01 ) ( 71 ) Applicant : THEMIS MEDICARE LIMITED , A61K 31 / 167 ( 2006 . 01 ) Mumbai (IN ) A61K 31 / 57 (2006 . 01) (52 ) U . S . CI. (72 ) Inventors : Dinesh Shantilal PATEL , Mumbai CPC .. .. .. A61K 47 / 10 ( 2013 . 01 ) ; A61K 9 /4858 ( IN ) ; Sachin Dinesh PATEL , Mumbai ( 2013 .01 ) ; A61K 9 /08 ( 2013 .01 ) ; A61K 9 / 06 ( IN ) ; Shashikant Prabhudas ( 2013 .01 ) ; A61K 9 / 0014 ( 2013 .01 ) ; A61K KURANI, Mumbai ( IN ) ; Madhavlal 31/ 573 ( 2013 .01 ) ; A61K 31 /57 ( 2013 .01 ) ; Govindlal PATEL , Mumbai ( IN ) A61K 31/ 565 ( 2013 .01 ) ; A61K 31 /4439 (73 ) Assignee : THEMIS MEDICARE LIMITED , ( 2013 .01 ) ; A61K 31/ 167 ( 2013 .01 ) ; A61K Mumbai (IN ) 9 /0048 ( 2013 .01 ) ; A61K 9 /0019 (2013 .01 ) ( 57 ) ABSTRACT (21 ) Appl. No .: 15 / 801, 390 The present invention relates to pharmaceutical composi tions of various pharmaceutical actives, especially lyophilic ( 22 ) Filed : Nov . 2 , 2017 and hydrophilic actives containing Diethylene glycol mono ethyl ether or other alkyl derivatives thereof as a primary Related U . S . Application Data vehicle and /or to pharmaceutical compositions utilizing (62 ) Division of application No. 14 /242 , 973 , filed on Apr. Diethylene glycol monoethyl ether or other alkyl derivatives 2 , 2014 , now Pat. No. 9 , 827 ,315 . -
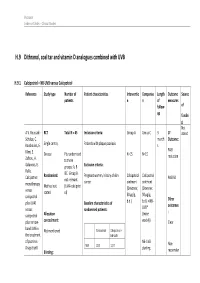
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL Et Al
US 20140296.191A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0296.191 A1 PATEL et al. (43) Pub. Date: Oct. 2, 2014 (54) COMPOSITIONS OF PHARMACEUTICAL (52) U.S. Cl. ACTIVES CONTAINING DETHYLENE CPC ............... A61K 47/10 (2013.01); A61 K9/0019 GLYCOL MONOETHYLETHER OR OTHER (2013.01); A61 K9/0048 (2013.01); A61 K ALKYL DERVATIVES 45/06 (2013.01) USPC ........... 514/167: 514/177; 514/178: 514/450; (71) Applicant: THEMIS MEDICARE LIMITED, 514/334: 514/226.5: 514/449; 514/338; Mumbai (IN) 514/256; 514/570; 514/179; 514/174: 514/533; (72) Inventors: Dinesh Shantilal PATEL, Mumbai (IN); 514/629; 514/619 Sachin Dinesh PATEL, Mumbai (IN); Shashikant Prabhudas KURANI, Mumbai (IN); Madhavlal Govindlal (57) ABSTRACT PATEL, Mumbai (IN) (73) Assignee: THEMIS MEDICARE LIMITED, The present invention relates to pharmaceutical compositions Mumbai (IN) of various pharmaceutical actives, especially lyophilic and hydrophilic actives containing Diethylene glycol monoethyl (21) Appl. No.: 14/242,973 ether or other alkyl derivatives thereofas a primary vehicle and/or to pharmaceutical compositions utilizing Diethylene (22) Filed: Apr. 2, 2014 glycol monoethyl ether or other alkyl derivatives thereofas a primary vehicle or as a solvent system in preparation of Such (30) Foreign Application Priority Data pharmaceutical compositions. The pharmaceutical composi Apr. 2, 2013 (IN) ......................... 1287/MUMA2013 tions of the present invention are safe, non-toxic, exhibits enhanced physical stability compared to conventional formu Publication Classification lations containing such pharmaceutical actives and are Suit able for use as injectables for intravenous and intramuscular (51) Int. Cl. administration, as well as for use as a preformed solution/ A647/ (2006.01) liquid for filling in and preparation of capsules, tablets, nasal A6 IK 45/06 (2006.01) sprays, gargles, dermal applications, gels, topicals, liquid oral A6 IK9/00 (2006.01) dosage forms and other dosage forms. -

Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives
pharmaceuticals Article Simulation of Physicochemical and Pharmacokinetic Properties of Vitamin D3 and Its Natural Derivatives Subrata Deb * , Anthony Allen Reeves and Suki Lafortune Department of Pharmaceutical Sciences, College of Pharmacy, Larkin University, Miami, FL 33169, USA; [email protected] (A.A.R.); [email protected] (S.L.) * Correspondence: [email protected] or [email protected]; Tel.: +1-224-310-7870 or +1-305-760-7479 Received: 9 June 2020; Accepted: 20 July 2020; Published: 23 July 2020 Abstract: Vitamin D3 is an endogenous fat-soluble secosteroid, either biosynthesized in human skin or absorbed from diet and health supplements. Multiple hydroxylation reactions in several tissues including liver and small intestine produce different forms of vitamin D3. Low serum vitamin D levels is a global problem which may origin from differential absorption following supplementation. The objective of the present study was to estimate the physicochemical properties, metabolism, transport and pharmacokinetic behavior of vitamin D3 derivatives following oral ingestion. GastroPlus software, which is an in silico mechanistically-constructed simulation tool, was used to simulate the physicochemical and pharmacokinetic behavior for twelve vitamin D3 derivatives. The Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) Predictor and PKPlus modules were employed to derive the relevant parameters from the structural features of the compounds. The majority of the vitamin D3 derivatives are lipophilic (log P values > 5) with poor water solubility which are reflected in the poor predicted bioavailability. The fraction absorbed values for the vitamin D3 derivatives were low except for calcitroic acid, 1,23S,25-trihydroxy-24-oxo-vitamin D3, and (23S,25R)-1,25-dihydroxyvitamin D3-26,23-lactone each being greater than 90% fraction absorbed. -

Dietary Supplements Compendium Volume 1
2015 Dietary Supplements Compendium DSC Volume 1 General Notices and Requirements USP–NF General Chapters USP–NF Dietary Supplement Monographs USP–NF Excipient Monographs FCC General Provisions FCC Monographs FCC Identity Standards FCC Appendices Reagents, Indicators, and Solutions Reference Tables DSC217M_DSCVol1_Title_2015-01_V3.indd 1 2/2/15 12:18 PM 2 Notice and Warning Concerning U.S. Patent or Trademark Rights The inclusion in the USP Dietary Supplements Compendium of a monograph on any dietary supplement in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of, or authority to exercise, any right or privilege protected by such patent or trademark. All such rights and privileges are vested in the patent or trademark owner, and no other person may exercise the same without express permission, authority, or license secured from such patent or trademark owner. Concerning Use of the USP Dietary Supplements Compendium Attention is called to the fact that USP Dietary Supplements Compendium text is fully copyrighted. Authors and others wishing to use portions of the text should request permission to do so from the Legal Department of the United States Pharmacopeial Convention. Copyright © 2015 The United States Pharmacopeial Convention ISBN: 978-1-936424-41-2 12601 Twinbrook Parkway, Rockville, MD 20852 All rights reserved. DSC Contents iii Contents USP Dietary Supplements Compendium Volume 1 Volume 2 Members . v. Preface . v Mission and Preface . 1 Dietary Supplements Admission Evaluations . 1. General Notices and Requirements . 9 USP Dietary Supplement Verification Program . .205 USP–NF General Chapters . 25 Dietary Supplements Regulatory USP–NF Dietary Supplement Monographs .