A Randomised Clinical Study of Alfacalcidol and Paricalcitol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Vitamin D and Its Analogues Decrease Amyloid- (A) Formation
International Journal of Molecular Sciences Article Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation Marcus O. W. Grimm 1,2,3,*,† ID , Andrea Thiel 1,† ID , Anna A. Lauer 1 ID , Jakob Winkler 1, Johannes Lehmann 1,4, Liesa Regner 1, Christopher Nelke 1, Daniel Janitschke 1,Céline Benoist 1, Olga Streidenberger 1, Hannah Stötzel 1, Kristina Endres 5, Christian Herr 6 ID , Christoph Beisswenger 6, Heike S. Grimm 1 ID , Robert Bals 6, Frank Lammert 4 and Tobias Hartmann 1,2,3 1 Experimental Neurology, Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany; [email protected] (A.T.); [email protected] (A.A.L.); [email protected] (J.W.); [email protected] (J.L.); [email protected] (L.R.); [email protected] (C.N.); [email protected] (D.J.); [email protected] (C.B.); [email protected] (O.S.); [email protected] (H.S.); [email protected] (H.S.G.); [email protected] (T.H.) 2 Neurodegeneration and Neurobiology, Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany 3 Deutsches Institut für DemenzPrävention (DIDP), Saarland University, Kirrberger Str. 1, 66421 Homburg/Saar, Germany 4 Department of Internal Medicine II–Gastroenterology, Saarland University Hospital, Saarland University, Kirrberger Str. 100, 66421 Homburg/Saar, Germany; [email protected] 5 Department of Psychiatry and Psychotherapy, Clinical Research Group, University Medical Centre Johannes Gutenberg, University of Mainz, Untere Zahlbacher Str. 8, 55131 Mainz, Germany; [email protected] 6 Department of Internal Medicine V–Pulmonology, Allergology, Respiratory Intensive Care Medicine, Saarland University Hospital, Kirrberger Str. -

A Clinical Update on Vitamin D Deficiency and Secondary
References 1. Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic 17. Ennis JL, Worcester EM, Coe FL, Sprague SM. Current recommended 32. Thimachai P, Supasyndh O, Chaiprasert A, Satirapoj B. Efficacy of High 38. Kramer H, Berns JS, Choi MJ, et al. 25-Hydroxyvitamin D testing and kidney disease. Clin J Am Soc Nephrol. 2008;3:1144-1151. 25-hydroxyvitamin D targets for chronic kidney disease management vs. Conventional Ergocalciferol Dose for Increasing 25-Hydroxyvitamin supplementation in CKD: an NKF-KDOQI controversies report. Am J may be too low. J Nephrol. 2016;29:63-70. D and Suppressing Parathyroid Hormone Levels in Stage III-IV CKD Kidney Dis. 2014;64:499-509. 2. Hollick MF. Vitamin D: importance in the prevention of cancers, type 1 with Vitamin D Deficiency/Insufficiency: A Randomized Controlled Trial. diabetes, heart disease, and osteoporosis. Am J Clin Nutr 18. OPKO. OPKO diagnostics point-of-care system. Available at: http:// J Med Assoc Thai. 2015;98:643-648. 39. Jetter A, Egli A, Dawson-Hughes B, et al. Pharmacokinetics of oral 2004;79:362-371. www.opko.com/products/point-of-care-diagnostics/. Accessed vitamin D(3) and calcifediol. Bone. 2014;59:14-19. September 2 2015. 33. Kovesdy CP, Lu JL, Malakauskas SM, et al. Paricalcitol versus 3. Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 40. Petkovich M, Melnick J, White J, et al. Modified-release oral calcifediol of vitamin D status and cancer incidence and mortality in men. -

A New Role for Vitamin D Receptor Activation in Chronic Kidney Disease
A NEW ROLE FOR VITAMIN D RECEPTOR ACTIVATION IN CHRONIC KIDNEY DISEASE José M Valdivielso 1, Jorge Cannata-Andía2, Blai Coll 1 and Elvira Fernández 1 1Laboratorio de Nefrología Experimental, Hospital Universitario Arnau de Vilanova, IRBLLEIDA, REDinREN ISCIII Lleida, Spain. 2Servicio de Metabolismo Óseo y Mineral. Hospital Universitario Central de Asturias. Instituto “Reina Sofía” de Investigación. REDinREN ISCIII. Universidad de Oviedo Running Title: New role of VDRA Correspondence to: Dr. José M Valdivielso, Laboratorio de Nefrología Experimental, IRBLLEIDA Hospital Universitari Arnau de Vilanova Rovira Roure 80 25198 Lleida, Spain. Phone: +34 973003650 Fax: +34 973702213 e-mail: [email protected] Abstract Vitamin D has proven to be much more than a simple ‘calcium hormone’. The fact that the vitamin D receptor has been found in cells not related to mineral metabolism supports that statement. The interest of nephrologists in Vitamin D and its effects beyond mineral metabolism has increased over the last few years, evidencing the importance of this so-called ‘sunshine hormone’. In the present review, we highlight the most recent developments in the traditional use of vitamin D in chronic kidney disease (CKD) patients, namely the control of secondary hyperparathyroidism (sHPT). Furthermore, we also explore the data available regarding the new possible therapeutic uses of vitamin D for the treatment of other complications present in CKD patients, such as vascular calcification, left ventricular hypertrophy or proteinuria. Finally, some still scarce but very promising data regarding a possible role of vitamin D in kidney transplant patients are also reviewed. The available data point to a potential beneficial effect of vitamin D in CKD patients beyond the control of mineral metabolism. -
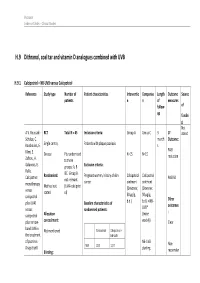
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

Vitamin D and Anemia: Insights Into an Emerging Association Vin Tangpricha, Emory University Ellen M
Vitamin D and anemia: insights into an emerging association Vin Tangpricha, Emory University Ellen M. Smith, Emory University Journal Title: Current Opinion in Endocrinology, Diabetes and Obesity Volume: Volume 22, Number 6 Publisher: Lippincott, Williams & Wilkins | 2015-12-01, Pages 432-438 Type of Work: Article | Post-print: After Peer Review Publisher DOI: 10.1097/MED.0000000000000199 Permanent URL: https://pid.emory.edu/ark:/25593/rtnx3 Final published version: http://dx.doi.org/10.1097/MED.0000000000000199 Copyright information: © 2015 Wolters Kluwer Health, Inc. All rights reserved. Accessed September 30, 2021 7:39 AM EDT HHS Public Access Author manuscript Author Manuscript Author ManuscriptCurr Opin Author Manuscript Endocrinol Diabetes Author Manuscript Obes. Author manuscript; available in PMC 2016 December 01. Published in final edited form as: Curr Opin Endocrinol Diabetes Obes. 2015 December ; 22(6): 432–438. doi:10.1097/MED. 0000000000000199. Vitamin D and Anemia: Insights into an Emerging Association Ellen M. Smith1 and Vin Tangpricha1,2,3 1Nutrition and Health Sciences Graduate Program, Laney Graduate School, Emory University, Atlanta, GA, USA 2Division of Endocrinology, Metabolism, and Lipids, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA 3Atlanta VA Medical Center, Decatur, GA, USA Abstract Purpose of review—This review highlights recent findings in the emerging association between vitamin D and anemia through discussion of mechanistic studies, epidemiologic studies, and clinical trials. Recent findings—Vitamin D has previously been found to be associated with anemia in various healthy and diseased populations. Recent studies indicate that the association may differ between race and ethnic groups and is likely specific to anemia of inflammation. -

Paricalcitol | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Paricalcitol This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Zemplar Brand Names: Canada Zemplar What is this drug used for? It is used to treat high parathyroid hormone levels in certain patients. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: High calcium levels or high vitamin D levels. This is not a list of all drugs or health problems that interact with this drug. Paricalcitol 1/8 Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this drug with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor. What are some things I need to know or do while I take this drug? All products: Tell all of your health care providers that you take this drug. This includes your doctors, nurses, pharmacists, and dentists. Have blood work checked as you have been told by the doctor. Talk with the doctor. If you are taking other sources of vitamin D, talk with your doctor. -

Specialty Guideline Management Crysvita
Effective Date: 12/2019 Reviewed: 9/2019, 6/2020, 1/2021, 5/2021 Scope: Medicaid SPECIALTY GUIDELINE MANAGEMENT CRYSVITA (burosumab-twza) POLICY I. CRITERIA FOR INITIAL APPROVAL X-linked hypophosphatemia A 6-month authorization may be granted for treatment of X-linked hypophosphatemia (XLH) when all of the following criteria are met: 1. Diagnosis of XLH confirmed by at least one of the following: a. Serum fibroblast growth factor-23 (FGF23) level > 30 pg/mL (>230 RU/mL in children 3 months-17 years; >180 RU/mL in adults using EDTA plasma); OR b. Phosphate regulating gene with homology to endopeptidases located on the X chromosome (PHEX-gene) mutations in the patient 2. Member is at least 6 months of age. 3. Member will not receive oral phosphate and/or active vitamin D analogs within 1 week prior to the start of therapy. 4. Adult patients must have had an inadequate response from oral phosphate and active vitamin D analogs. 5. For adults, dose requested is 1 mg/kg, rounded to nearest 10mg, every 4 weeks and dose does not exceed 90mg [Member’s weight must be provided]. 6. For pediatric members, dose requested is 0.8 mg/kg, rounded to nearest 10mg, every 2 weeks and dose does not exceed 90mg [Member’s weight must be provided]. 7. Baseline fasting serum phosphorus* level with current hypophosphatemia, defined as a phosphate level below the lower limit of the laboratory normal reference range (Note: serum phosphorus levels should be monitored periodically throughout therapy, required on renewal). 8. Must be prescribed by, or in consultation with, a nephrologist or endocrinologist. -

New Roles for Vitamin D Superagonists: from COVID to Cancer
REVIEW published: 31 March 2021 doi: 10.3389/fendo.2021.644298 New Roles for Vitamin D Superagonists: From COVID to Cancer David J. Easty 1, Christine J. Farr 2* and Bryan T. Hennessy 3,4 1 Department of Medical Oncology, Our Lady of Lourdes Hospital, Drogheda, Ireland, 2 Department of Genetics, University of Cambridge, Cambridge, United Kingdom, 3 Department of Molecular Medicine, Royal College of Surgeons in Ireland, Dublin, Ireland, 4 Department of Oncology, Our Lady of Lourdes Hospital, Drogheda, Ireland Vitamin D is a potent steroid hormone that induces widespread changes in gene expression and controls key biological pathways. Here we review pathophysiology of vitamin D with particular reference to COVID-19 and pancreatic cancer. Utility as a therapeutic agent is limited by hypercalcemic effects and attempts to circumvent this problem have used vitamin D superagonists, with increased efficacy and reduced calcemic effect. A further caveat is that vitamin D mediates multiple diverse effects. Some of these (anti-fibrosis) are likely beneficial in patients with COVID-19 and pancreatic cancer, whereas others (reduced immunity), may be beneficial through attenuation of the Edited by: cytokine storm in patients with advanced COVID-19, but detrimental in pancreatic cancer. Jeff M. P. Holly, University of Bristol, United Kingdom Vitamin D superagonists represent an untapped resource for development of effective Reviewed by: therapeutic agents. However, to be successful this approach will require agonists with William B. Grant, high cell-tissue specificity. Sunlight Nutrition and Health Research Center, United States Keywords: COVID-19, pancreatic cancer, pancreatic stellate cell, superagonist, vitamin D, paricalcitol Erick Legrand, Centre Hospitalier Universitaire d’Angers, France *Correspondence: INTRODUCTION Christine J. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Paricalcitol Versus Calcitriol in the Treatment of Secondary Hyperparathyroidism
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 63 (2003), pp. 1483–1490 Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism STUART M. SPRAGUE,FRANCISCO LLACH,MICHAEL AMDAHL,CAROL TACCETTA, and DANIEL BATLLE Division of Nephrology/Hypertension and Department of Medicine, Northwestern University Feinberg School of Medicine, Evanston and Chicago, Illinois; Georgetown University Medical School, Washington, D.C.; and Abbott Laboratories, North Chicago, Illinois Paricalcitol versus calcitriol in the treatment of secondary hyper- Osteitis fibrosa cystica, resulting from poorly con- parathyroidism. trolled secondary hyperparathyroidism, remains a com- Background. Management of secondary hyperparathyroid- mon manifestation of renal osteodystrophy and causes ism has included the use of active vitamin D or vitamin D ana- logs for the suppression of parathyroid hormone (PTH) secre- significant morbidity in patients with chronic renal fail- tion. Although, these agents are effective, therapy is frequently ure [1, 2]. Decreased renal production of calcitriol (1,25 limited by hypercalcemia, hyperphosphatemia, and/or eleva- vitamin D ), hypocalcemia, and hyperphosphatemia are ϫ 3 tions in the calcium-phosphorus (Ca P) product. In clinical the major contributing factors to the development of studies, paricalcitol was shown to be effective at reducing PTH concentrations without causing significant hypercalcemia or secondary hyperparathyroidism [1, 3–5]. Calcitriol is syn- hyperphosphatemia as compared to placebo. A comparative thesized from previtamin D3 by hydroxylation on carbons study was undertaken in order to determine whether paricalci- 25 and 1 in the liver and kidney, respectively [6]. The tol provides a therapeutic advantage to calcitriol. -

Vitamin D Analog (Oral) Step Therapy Program
STEP THERAPY POLICY POLICY: Vitamin D Analog (Oral) Step Therapy Program APPROVAL DATE: 08/07/2019 DRUGS AFFECTED: • Hectorol® (doxercalciferol capsules – Genzyme; generics) Rayaldee® (calcifediol extended-release capsule – OPKO) Rocaltrol® (calcitriol capsules and oral solution – Validus; generics) Zemplar® (paricalcitol capsules – Abbvie; generics) OVERVIEW Vitamin D analogs are therapeutic options for the reduction of elevated parathyroid hormone (PTH) levels in patients with chronic kidney disease (CKD) who have secondary hyperparathyroidism (SHPT).1 SHPT is a common complication of CKD, affecting nearly all of the more than 400,000 patients receiving dialysis (CKD Stage 5D classification) in the US.2,3 It may also affect CKD patients not yet on dialysis.2,3 SHPT is associated with increased PTH levels and alterations in calcium and phosphorus levels.2 These alterations can then lead to bone disease, bone pain and fractures, and vascular and soft tissue calcifications which may lead to cardiovascular (CV) morbidity and mortality. Exogenous calcitriol is a synthetic vitamin D analog, which is active in the regulation of the absorption of calcium from the gastrointestinal (GI) tract and its utilization in the body.4,7 Doxercalciferol is a synthetic vitamin D2 analog which undergoes metabolic activation in vivo to form 1α,25-(OH)2D2, a 5 naturally occurring biologically active form of vitamin D2. Paricalcitol is a synthetically manufactured analog of calcitriol.6 Rayaldee is a synthetically manufactured prohormone of calcitriol, calcifediol, which is also known as calcidiol.7 Calcifediol/calcidiol is converted to calcitriol in the kidney; calcitriol then binds to vitamin D receptors (VDRs) in the target tissues.