SCANDIUM in BIOTITE AS a GEOLOGIC THERMOMETER °/O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fylkesmannens Kommunebilde Evje Og Hornnes Kommune 2013
Fylkesmannens kommunebilde Evje og Hornnes kommune 2013 Innhold 1 Innledning 3 2 Planlegging og styring av kommunen 4 2.1 Demografi 4 2.2 Likestilling 5 2.3 Økonomi og økonomistyring 8 2.4 Bosetting og kvalifisering av flyktninger 12 3 Tjenesteyting og velferdsproduksjon 14 3.1 Barnehage 14 3.2 Tidlig innsats 18 3.3 Utdanning 19 3.4 Vergemål 25 3.5 Byggesak 26 3.6 Folkehelse og levekår 27 3.7 Barneverntjenesten 29 3.8 Helsesøster- og jordmortjeneste 32 3.9 Helsetjenester 33 3.10 Tjenester i sykehjem 35 3.11 Velferdsprofil 37 3.12 Arealplanlegging 39 3.13 Forurensning 40 3.14 Landbruk 41 Side: 2 - Fylkesmannen i Aust-Agder, kommunebilde 2013 Evje og Hornnes 1 Innledning Kommunebilde, som Fylkesmannen utarbeider for alle 15 kommuner i Aust-Agder, er et viktig element i styringsdialogen mellom kommunene og Fylkesmannen. Det gir ikke et fullstendig utfyllende bilde av kommunens virksomhet, men gjelder de felt der Fylkesmannen har oppdrag og dialog mot kommunene. Det er et dokument som peker på utfordringer og handlingsrom. Fylkesmannen er opptatt av at kommunene kan løse sine oppgaver best mulig og ha politisk og administrativt handlingsrom. Det er viktig at kommunene har et best mulig beslutningsgrunnlag for de valg de tar. Blant annet derfor er kommunebilde et viktig dokument. Kommunebilde er inndelt i tre 1. Innledning 2. Planlegging og styring av kommunen 3. Tjenesteyting og velferdsproduksjon Under hvert punkt har vi oppsummert Fylkesmannens vurdering av utfordringspunkter for kommunen. Dette er punkter vi anbefaler kommunen å legge til grunn i videre kommunalplanlegging. Kommunebilde er et godt utgangspunkt for å få et makrobilde av kommunen og bør brukes aktivt i kommunen – ikke minst av de folkevalgte. -

Farsund & Listalandet
Live Camera CITY HARBOUR [Farsund2000] [GPS] [Photo-PostCard-NEW] [HELP] [About us] [NewsLetter] [Tell-A-Friend] [GuestHarbour ] [Weather] [PhotoGallery] [Members] [Forening / Lag] [©CopyRight] [Admin] - Chose County - Chose Counsil N -Chose AirPort.No Velkommen til VisitEurope.NO Akershus Agdenes Alta Airport Aust-Agder Alstahaug Andøya Her kan din bedrift profileres med både oppføringer og bannere til svært Buskerud Alta Bardufoss gunstige priser og betingelser. Portalen er tilknyttet den europeiske Finnmark Alvdal Båtsfjord hovedportalen VisitEurope.TV som representerer alle land i Europa. Vi inkluderer gode og viktige funksjonaliteter uten ekstra betaling. Bestill nå eller få mer informasjon ved å klikke på overskriften. Velkomm channel: - Turn ON Radio -------> Farsund & Listalandet 1200 EPostCards/Photo's Welcome to Farsund! The municipality of Farsund has ca. 9.200 inhabitants, mainly concentrated on three centres of population - Farsund town, Vanse and Vestbygda. It also includes the outlying districts of Lista, Herad and Spind. Shipping, fishing and agriculture have been the main industries in the area. Today Farsund is the largest agrcultural district in the county of Vest-Agder, having 26 km2 productive land, 88 km2 forest and 17 k m2 freshwater areas. Farsund was already recognized as a trading centre in 1795, and in 1995 celebrated its 200-years jubilee with town status. Vanse: was formerly the largest centre of population in the district. Today it has 2.500 inhabitants, and some of the council offices are still situated there. Vestbygda: is built round the only harbour of any size in a particularly exposed stretch of the coast. There was a considerable emigration to the United States from this region in former times. -

How Uniform Was the Old Norse Religion?
II. Old Norse Myth and Society HOW UNIFORM WAS THE OLD NORSE RELIGION? Stefan Brink ne often gets the impression from handbooks on Old Norse culture and religion that the pagan religion that was supposed to have been in Oexistence all over pre-Christian Scandinavia and Iceland was rather homogeneous. Due to the lack of written sources, it becomes difficult to say whether the ‘religion’ — or rather mythology, eschatology, and cult practice, which medieval sources refer to as forn siðr (‘ancient custom’) — changed over time. For obvious reasons, it is very difficult to identify a ‘pure’ Old Norse religion, uncorroded by Christianity since Scandinavia did not exist in a cultural vacuum.1 What we read in the handbooks is based almost entirely on Snorri Sturluson’s representation and interpretation in his Edda of the pre-Christian religion of Iceland, together with the ambiguous mythical and eschatological world we find represented in the Poetic Edda and in the filtered form Saxo Grammaticus presents in his Gesta Danorum. This stance is more or less presented without reflection in early scholarship, but the bias of the foundation is more readily acknowledged in more recent works.2 In the textual sources we find a considerable pantheon of gods and goddesses — Þórr, Óðinn, Freyr, Baldr, Loki, Njo3rðr, Týr, Heimdallr, Ullr, Bragi, Freyja, Frigg, Gefjon, Iðunn, et cetera — and euhemerized stories of how the gods acted and were characterized as individuals and as a collective. Since the sources are Old Icelandic (Saxo’s work appears to have been built on the same sources) one might assume that this religious world was purely Old 1 See the discussion in Gro Steinsland, Norrøn religion: Myter, riter, samfunn (Oslo: Pax, 2005). -

Sluttrapport Fra Forvaltningskontroll Landbruk Evje Og Hornnes Og Iveland
Landbruksavdelingen Sluttrapport fra kontroll av forvaltningen av utvalgte tilskuddsordninger i landbruket – 2016 Kontrollert Evje og Hornnes og Iveland Kontrollpersoner fra Hans Bach- enhet kommuner Fylkesmannen i Evensen og Rolf Aust- og Vest-Agder Inge Pettersen Kontrollomfang Dato for sluttrapport 01.12.2016 -Avløsning ved sykdom og fødsel mv. - Tilskudd til spesielle miljøtiltak i landbruket (SMIL). Kommune Kontaktperson Evje og Hornnes og Iveland kommune Inge Eftevand Rapportens innhold Rapporten er utarbeidet etter gjennomført forvaltningskontroll i Iveland og Evje og Hornnes kommune innen ordningene med avløsning ved sykdom og fødsel mv. og tilskudd til spesielle miljøtiltak i landbruket (SMIL). Rapporten er utarbeidet som 1 rapport til begge kommunene siden det er felles forvaltning på de aktuelle landbrukstilskuddene. Den beskriver eventuelle avvik og merknader som ble avdekket innen de kontrollerte områdene. Avvik er mangel på oppfyllelse av krav fastsatt i lov, forskrift og/eller rundskriv. Merknad er forhold som ikke er i strid med krav, men der Fylkesmannen finner grunn til å påpeke behov for forbedring. Bakgrunn for kontrollen Fylkesmannen har det regionale ansvar for å sikre at statlige midler brukes og forvaltes i samsvar med forutsetningene. Forvaltningskontrollen har hjemmelsgrunnlag i Økonomireglementet i staten (§ 15) og bakgrunn i fullmaktsbrev fra Landbruksdirektoratet for 2016. Ved å avdekke eventuelle avvik og brudd på regelverk og rutiner vil dette kunne hjelpe kommunen fram mot en sikrere, bedre og mer effektiv forvaltning av tilskuddsordningene. Gjennomføring og omfang Kontrollen ble gjennomført som en dokumentkontroll. Fylkesmannen gjennomgikk forhåndsvalgte saker som kommunene hadde oversendt. Dette gjaldt 3 saker om tilskudd til avløsning til sykdom og fødsel mv. og 4 saker tilknyttet SMIL ordningen. -

Risiko- Og Vesentlighetsvurdering: Iveland Kommune Har Etiske Retningslinjer Fra 2017 Som Tar for Seg En Rekke Tema, Herunder Varsling
Risiko- og vesentlighetsvurdering: Forvaltningsrevisjon og eierskapskontroll Iveland kommune Valgperioden 2019-2023 Kristiansand, September 2020 Agder Kommunerevisjon IKS E-post: [email protected] Avd. Setesdal Postadr.: Postboks 4 Hjemmeside: www.agderkomrev.no Kasernevegen 19 4685 Nodeland Telefon Kr.sand: 38 07 27 00 4735 Evje Hovedktr. Tollbodgata 37 Telefon Evje: 977 60 455 Org.nr. 987 183 918 Risiko- og vesentlighetsvurdering for Iveland kommune INNHOLDSFORTEGNELSE FORORD .................................................................................................................................... 2 INNHOLDSFORTEGNELSE ................................................................................................... 3 1. INNLEDNING .................................................................................................................... 5 1.1 Kontrollutvalgets bestilling ......................................................................................... 5 1.2 Hva er forvaltningsrevisjon? ....................................................................................... 5 1.3 Hva er eierskapskontroll? ............................................................................................ 6 1.4 Forholdet mellom forvaltningsrevisjon og eierskapskontroll ...................................... 6 1.5 Om risiko- og vesentlighetsvurderingen ...................................................................... 7 1.6 Avgrensninger og analytiske begrensninger ............................................................... -

Sparebanken Sør Boligkreditt AS
Sparebanken Sør Boligkreditt AS Investor Presentation March 2016 Executive summary . The fifth largest savings bank in Norway with a strong market position in Southern Norway . High capitalization; Capital ratio of 15.5 % - Core Tier 1 ratio of 12.7 % at December 31th 2015 Sparebanken Sør . Rated A1 (stable outlook) by Moody’s . Strong asset quality – 65 per cent of loan book to retail customers . New rights issue of NOK 600 million in progress will strengthen capital ratio even further . 100 % owned and dedicated covered bond subsidiary of Sparebanken Sør . Cover pool consisting of 100 % prime Norwegian residential mortgages Sparebanken Sør . High quality cover pool reflected by the weighted average LTV of 55.3 % Boligkreditt . Covered bonds rated Aaa by Moody’s with 5 notches of “leeway” . Strong legal framework for covered bonds in Norway with LTV limit of 75 % for residential mortgages . Low interest rates, weak NOK and fiscal stimulus are counterbalancing economic slowdown stemming from the lower oil investments . Unemployment is expected to increase gradually but from a very low level and the unemployment Norwegian rate remains well below the European levels economy . The Norwegian government has a strong financial position with large budget surplus. The government pension fund, which accounts over 200% of the GDP, provides the government with substantial economic leeway . The Southern region is clearly less exposed to oil production than Western Norway Southern region . Registered unemployment in the Southern region remains below -

Områdeplan Med KU Grovane I Vennesla Og Iveland Kommuner
PLANINITIATIV Områdeplan med KU Grovane i Vennesla og Iveland kommuner Kilde: www.googlemaps.no Kunde: Otra Holding AS Prosjekt: «Grovane» næringspark - reguleringsplan Prosjektnummer: 10220770 Rev.: 0 Dato: 5/11-2020 Innholdsfortegnelse 1 Innledning ...................................................................................................................... 3 2 Formålet med planen ..................................................................................................... 3 3 Planområdet og virkninger utenfor planområdet ............................................................ 3 3.1 Forslag til plangrense ......................................................................................................................... 3 4 Planlagt bebyggelse, anlegg og andre tiltak .................................................................. 4 4.1 Utbyggingsvolum og byggehøyder .................................................................................................... 4 4.2 Funksjonell og miljømessig kvalitet .................................................................................................... 4 5 Tiltakets virkning på, og tilpasning til omgivelser ........................................................... 4 6 Forholdet til andre planer ............................................................................................... 6 7 Medvirkningsprosesser / interessekonflikter .................................................................. 7 8 Temaer som skal avklares i planbeskrivelsen -

Lars Aase.Pdf (1.232Mb)
MIGRASJON, MISJON OG MAMMON EN NÆRSTUDIE AV SØRLANDSK UTVANDRING TIL SØR-AFRIKA I PERIODEN 1849-1920. LARS AASE Masteroppgaven er gjennomført som ledd i utdanningen ved Universitetet i Agder og er godkjent som del av denne utdanningen. Denne godkjenningen innebærer ikke at universitetet innestår for de metoder som er anvendt og de konklusjoner som er trukket. Universitetet i Agder, 2010 Fakultet for Humaniora og pedagogikk Institutt for religion, filosofi og historie Innhold Innhold ................................................................................................................................... 2 Forord ..................................................................................................................................... 4 Abstract ...................................................................................................................................... 5 Kapittel 1 ~ Innledning............................................................................................................... 7 Problemstilling og avgrensninger ........................................................................................... 7 Norsk emigrasjonsforskning .................................................................................................. 8 ”Det norske eksodus” ............................................................................................................. 9 Sørlandsk utvandring ........................................................................................................... -

Folketellingen 1. November 1960. 0937 Evje Og Hornnes
OKEEIGE 1 OEME 19 llnrlttr lbånd tll -- rnr EE OG OES 937 SAISISK SEAYÅ - OSO MEØE I KØ OG AEE I seie "eigsesuae -- iake;; ee ;11 - ogose" egge Saisisk Seayå am e ikigse esuae a okeeige 19 o e eke kommue I iegg a e også a me okea a i- igee eige og gi e ogose oe okea og aessammeseig o e 5-å am i 19 0 uiseige ske før e mes eaee aee a eige oe- igge aee i isse eee må eo eakes som oeøige å e gee kae og aeee ø e meke seg øgee Kae ^ o e eeskommue a e a i e ka som øs og ems a i omå å ise oeige a kommue i eigskese e e- igskes a å si ege umme Ae eyge søk e ski u som ege e11igkese sik a ige kes omae åe eeyggese og seyge søk e øyakige agesig a e eyge kesee a e ikke æ muig å gegi å kae E a ae make a e kes e eyg e A age e rød sike u ummee å kese å sie 3 e eigskesee agi egee å kae a øgee eyig ykk ø sek - kommuegese y ø sek - eigskesgese ykk sa sek w kysie y sa sek - e Skaeig makee sø isø ø sike u eigskeses umme makee a ee kese e eyg (se oeo Kommuegesee e ege i sik e a 1/11 19 aee e 1 okemege e okeeigee I aee oe okemege e okeeigee a e geee a sike å å gi a iake i 11 aee a eigee 11 - 15 e ee a uikasoe "okemaegea eegese 15 - 15" aee a e seee eigee e ee a e esekie eigsuikasoee Oe e så ag isom a e imiei ske så mage eige i kommueieige a e o e ese kommue ikke ka gis e amøa 2- tallserie for hele perioden. -
Accession Number Title Author Publisher Year Source 42000
Accession Number Title Author Publisher Year Source 42000 Lucas County, Ohio Chancery Records 1836-1853 Index to Litigants/Beverly Todd Reed Lucas Co. Chapter OGS 1991 Lucas Co. Chapter OGS Military Pension Applications 1875-1889 Buchanan & MacGahan Law Firm Toledo, Ohio/Beverly Todd 42001 Reed Beverly Todd Reed 1997 Lucas Co. Chapter OGS Military Pension Applications 1875-1889 Buchanan & MacGahan Law Firm Toledo, Ohio/Beverly Todd 42002 Reed Beverly Todd Reed 1997 Lucas Co. Chapter OGS 42003 Arise Wild Land, As We Were In Milton Township, Ohio/Lindsey Williams Atkinson Printing 1982 Ali Burger My Dear Hindalla Remember Me, Letters from a Lost World May 1937 - January 1940/Marlene S. Windjammer Adventure 42004 Englander Publishing 2012 Marlene Saul Englander 42005 The Wittkop Family, In Search of the Sisters Wittkp/David A. Wittkop, Gerald C. Wittkop Dandelion Acre Productions 2004 Virginia Cassady 42006 The Wittkop Family, In Search of the Brothers Wittkop/David A. Wittkop, Gerald C. Wittkop Dandelion Acre Productions 2000 Virginia Cassady History and Genealogy: Finding Clues to Ancestral Lives - OGS 2012 Conference Syllabus/Ohio 42007 Genealogical Society Ohio Genealogical Society 2012 Tom Neel 42008 The Family of Zadock Hawkins/Lynn E. Garn, Ronnie E. Hawkins, Sr., G. Christian Larsen Lynn E. Garn 2005 Lynn Garn Your Family Tree, 42009 Before 1837, The Essential Guide/Adam Rees ancestry.co.uk 2012 Sunda Peters 42010 News Journal 100 Years of History, 1893-1992/Mansfield News Journal Mansfield News Journal 1992 Robert Cunning Estate 42011 1871Atlas of Knox County Ohio/JA Caldwell & JW Starr Mayhill Publications 1971 Robert Cunning Estate 42012 The Life of William McKinley/PF Collier & Son PF Collier & Son 1901 Robert Cunning Estate 42013 Glimpses of the World/John L. -

Folketeljinga 1801. Ny Bearbeiding
OGES OISIEE SAISIKK 13 OKEEIGA 11 Y EAEIIG OUAIO CESUS 11 SAISISK SEAYÅ CEA UEAU O SAISICS O OWAY OSO 19 IS -337-119- FØREORD Denne publikasjonen gir opplysningar om befolkninga i Noreg i 1801 slik dei er komne fram etter ei ny bearbeiding av teljingsmaterialet frå det aret. Publikasjonen ligg fOre kort tid etter at Statis- tisk Sentralbyrå i særskilt hefte har prenta gamle tabellmanuskript frå Folketeljinga 1769 som tidlegare for ein stor del ikkje har vore publiserte. Folketeljinga 1801 er den første nominative teljinga her i landet, dvs. ei teljing med oppgåver for kvar einskild namngjeven person. Teljingane før og også etter 1801, fram til 1865, skilde seg ut på denmZten at dei lokalt teljingsansvarlege berre laga samandrag for grupper av personar. Tanken om ei ny bearbeiding av det verdfulle grunnmaterialet frå 1801 vart drOfta i Byrået tidleg på 1950-talet. Av ulike grunnar vart det ikkje gjort noko meir med saka den gongen. I 1968 gjorde professor Knut Mykland på vegner av historikarar ved universiteta i Bergen og Oslo og Norges lærerhOgskoie i Trondheim opptak til eit mete med Byrået om same sak. Det var full semje om at ei ny bearbeiding av dette maeiae ettermoderne statistiske isi ie kunne gi langt betre innsyn i strukturen i den norske befolkninga på 17- og 18-hundretalet enn dei kunnskapane ein tidlegare hadde fått ut av teljinga. Det gjaldt til dømes fordelinga etter alder og ekteskapeleg status, etter leveveg og samansetjing i husstandar. Moderne teknisk utstyr ville og gjere det mogleg med fleire kryssgrupperingar av materialet. Ved ny bearbeiding kunne ein i tillegg betre enn fOr stette krava til statistisk kunnskap om små geografiske einingar. -
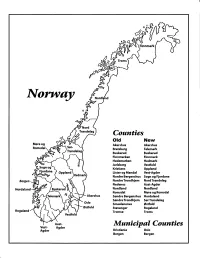
Norway Maps.Pdf
Finnmark lVorwny Trondelag Counties old New Akershus Akershus Bratsberg Telemark Buskerud Buskerud Finnmarken Finnmark Hedemarken Hedmark Jarlsberg Vestfold Kristians Oppland Oppland Lister og Mandal Vest-Agder Nordre Bergenshus Sogn og Fjordane NordreTrondhjem NordTrondelag Nedenes Aust-Agder Nordland Nordland Romsdal Mgre og Romsdal Akershus Sgndre Bergenshus Hordaland SsndreTrondhjem SorTrondelag Oslo Smaalenenes Ostfold Ostfold Stavanger Rogaland Rogaland Tromso Troms Vestfold Aust- Municipal Counties Vest- Agder Agder Kristiania Oslo Bergen Bergen A Feiring ((r Hurdal /\Langset /, \ Alc,ersltus Eidsvoll og Oslo Bjorke \ \\ r- -// Nannestad Heni ,Gi'erdrum Lilliestrom {", {udenes\ ,/\ Aurpkog )Y' ,\ I :' 'lv- '/t:ri \r*r/ t *) I ,I odfltisard l,t Enebakk Nordbv { Frog ) L-[--h il 6- As xrarctaa bak I { ':-\ I Vestby Hvitsten 'ca{a", 'l 4 ,- Holen :\saner Aust-Agder Valle 6rrl-1\ r--- Hylestad l- Austad 7/ Sandes - ,t'r ,'-' aa Gjovdal -.\. '\.-- ! Tovdal ,V-u-/ Vegarshei I *r""i'9^ _t Amli Risor -Ytre ,/ Ssndel Holt vtdestran \ -'ar^/Froland lveland ffi Bergen E- o;l'.t r 'aa*rrra- I t T ]***,,.\ I BYFJORDEN srl ffitt\ --- I 9r Mulen €'r A I t \ t Krohnengen Nordnest Fjellet \ XfC KORSKIRKEN t Nostet "r. I igvono i Leitet I Dokken DOMKIRKEN Dar;sird\ W \ - cyu8npris Lappen LAKSEVAG 'I Uran ,t' \ r-r -,4egry,*T-* \ ilJ]' *.,, Legdene ,rrf\t llruoAs \ o Kirstianborg ,'t? FYLLINGSDALEN {lil};h;h';ltft t)\l/ I t ,a o ff ui Mannasverkl , I t I t /_l-, Fjosanger I ,r-tJ 1r,7" N.fl.nd I r\a ,, , i, I, ,- Buslr,rrud I I N-(f i t\torbo \) l,/ Nes l-t' I J Viker -- l^ -- ---{a - tc')rt"- i Vtre Adal -o-r Uvdal ) Hgnefoss Y':TTS Tryistr-and Sigdal Veggli oJ Rollag ,y Lvnqdal J .--l/Tranbv *\, Frogn6r.tr Flesberg ; \.