Post-Traumatic Arthritis Following Intra-Articular Fractures: First Hit Or Chronic Overload?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Medical Policy Ultrasound Accelerated Fracture Healing Device
Medical Policy Ultrasound Accelerated Fracture Healing Device Table of Contents Policy: Commercial Coding Information Information Pertaining to All Policies Policy: Medicare Description References Authorization Information Policy History Policy Number: 497 BCBSA Reference Number: 1.01.05 Related Policies Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures, #498 Electrical Bone Growth Stimulation of the Appendicular Skeleton, #499 Bone Morphogenetic Protein, #097 Policy Commercial Members: Managed Care (HMO and POS), PPO, and Indemnity Members Low-intensity ultrasound treatment may be MEDICALLY NECESSARY when used as an adjunct to conventional management (i.e., closed reduction and cast immobilization) for the treatment of fresh, closed fractures in skeletally mature individuals. Candidates for ultrasound treatment are those at high risk for delayed fracture healing or nonunion. These risk factors may include either locations of fractures or patient comorbidities and include the following: Patient comorbidities: Diabetes, Steroid therapy, Osteoporosis, History of alcoholism, History of smoking. Fracture locations: Jones fracture, Fracture of navicular bone in the wrist (also called the scaphoid), Fracture of metatarsal, Fractures associated with extensive soft tissue or vascular damage. Low-intensity ultrasound treatment may be MEDICALLY NECESSARY as a treatment of delayed union of bones, including delayed union** of previously surgically-treated fractures, and excluding the skull and vertebra. 1 Low-intensity ultrasound treatment may be MEDICALLY NECESSARY as a treatment of fracture nonunions of bones, including nonunion*** of previously surgically-treated fractures, and excluding the skull and vertebra. Other applications of low-intensity ultrasound treatment are INVESTIGATIONAL, including, but not limited to, treatment of congenital pseudarthroses, open fractures, fresh* surgically-treated closed fractures, stress fractures, arthrodesis or failed arthrodesis. -

6.3.3 Distal Radius and Wrist
6 Specific fractures 6.3 Forearm and hand 6.3.3 Distal radius and wrist 1 Assessment 657 1.1 Biomechanics 657 1.2 Pathomechanics and classification 657 1.3 Imaging 658 1.4 Associated lesions 659 1.5 Decision making 659 2 Surgical anatomy 660 2.1 Anatomy 660 2.2 Radiographic anatomy 660 2.3 Preoperative planning 661 2.4 Surgical approaches 662 2.4.1 Dorsal approach 2.4.2 Palmar approach 3 Management and surgical treatment 665 3.1 Type A—extraarticular fractures 665 3.2 Type B—partial articular fractures 667 3.3 Type C—complete articular fractures 668 3.4 Ulnar column lesions 672 3.4.1 Ulnar styloid fractures 3.4.2 Ulnar head, neck, and distal shaft fractures 3.5 Postoperative care 674 3.6 Complications 676 3.7 Results 676 4 Bibliography 677 5 Acknowledgment 677 656 PFxM2_Section_6_I.indb 656 9/19/11 2:45:49 PM Authors Daniel A Rikli, Doug A Campbell 6.3.3 Distal radius and wrist of this stable pivot. The TFCC allows independent flexion/ 1 Assessment extension, radial/ulnar deviation, and pronation/supination of the wrist. It therefore plays a crucial role in the stability of 1.1 Biomechanics the carpus and forearm. Significant forces are transmitted across the ulnar column, especially while making a tight fist. The three-column concept (Fig 6.3.3-1) [1] is a helpful bio- mechanical model for understanding the pathomechanics of 1.2 Pathomechanics and classification wrist fractures. The radial column includes the radial styloid and scaphoid fossa, the intermediate column consists of the Virtually all types of distal radial fractures, with the exception lunate fossa and sigmoid notch (distal radioulnar joint, DRUJ), of dorsal rim avulsion fractures, can be produced by hyper- and the ulnar column comprises the distal ulna (DRUJ) with extension forces [2]. -

Treatment of Distal Radius Fractures – Clinical Outcome, Regional Variation and Health Economics
From THE DEPARTMENT OF CLINICAL SCIENCE AND EDUCATION, SÖDERSJUKHUSET Karolinska Institutet, Stockholm, Sweden TREATMENT OF DISTAL RADIUS FRACTURES – CLINICAL OUTCOME, REGIONAL VARIATION AND HEALTH ECONOMICS Jenny Saving Stockholm 2019 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Eprint AB 2019 © Jenny Saving, 2019 ISBN 978-91-7831-339-6 Treatment of distal radius fractures – clinical outcome, regional variation and health economics THESIS FOR DOCTORAL DEGREE (Ph.D.) By Jenny Saving, MD Principal Supervisor: Opponent: MD, Associate Professor Anders Enocson MD, Professor Lars Adolfsson Karolinska Institutet University of Linköping Department of Clinical Science and Education Department of Clinical and Experimental Division of Orthopaedics Medicine Södersjukhuset Examination Board: Co-supervisor(s): MD, Professor Hans Mallmin MD, PhD, Cecilia Mellstrand Navarro Uppsala University Karolinska Institutet Department of Surgical Sciences Department of Clinical Science and Education Section of Orthopaedics Division of Hand Surgery Södersjukhuset MD, Associate Professor Rüdiger Weiss Karolinska Institutet MD, Professor Sari Ponzer Department of Molecular Medicine and Surgery Karolinska Instiutet Karolinska University Hospital Department of Clinical Science and Education Division of Orthopaedics MD, Professor Olof Nilsson Södersjukhuset Uppsala University Department of Surgical Sciences Section of Orthopaedics To my family 3 4 ABSTRACT A distal radius fracture (DRF) remains the most common fracture encountered in health care. DRFs have traditionally been treated with a plaster or surgically with percutaneous methods. Since the end of the 20th century, when internal fixation with a volar locking plate (VLP) was introduced, the incidence of DRF surgery in general and of plating in particular have increased markedly. -

Metacarpal Fractures
METACARPAL FRACTURES BY LORYN P. WEINSTEIN, MD, AND DOUGLAS P. HANEL, MD The majority of metacarpal fractures are closed injuries amenable to conservative treatment with external immobilization and subsequent rehabilitation. Internal fixation is favored for unstable fracture patterns and patients who require early motion. Percutaneous pinning usually is successful for metacarpal neck fractures and comminuted head fractures. Shaft and base fractures can be treated with pinning or open reduction and internal fixation; the latter, being more rigid, allows early rehabilitation. External fixation has a limited yet defined role for metacarpal fractures with complex soft-tissue injury and/or segmental bone loss. The recent development of bioabsorbable implants holds promise for skeletal rigidity with minimal soft-tissue morbidity, but long-term in vivo data support- ing the use of these implants is not currently available. Copyright © 2002 by the American Society for Surgery of the Hand arly treatment of metacarpal fractures was lim- Surgical techniques rapidly expanded to include ret- ited to the only tools available: manipulation rograde fracture pinning, intramedullary pinning, and Eand casting. The discovery of percutaneous transfixion pinning. Many of the K-wires in use today fracture fixation near the turn of the century opened have the same diamond-shaped tip and sizing speci- up a new world of possibilities. It was 25 years after fications as the original design. Bennett’s original manuscript that Lambotte de- The first plate and screw set for the hand was scribed the first surgical stabilization of a basilar introduced in the late 1930s. By today’s standards, the thumb fracture by using a thin carpenter’s nail.1 By Hermann Metacarpal Bone Set was quite lean; it 1913, Lambotte had authored a fracture text with included 3 longitudinal plates of 2, 3, and 4 holes, a multiple examples of pinning, wiring, and plating of drill, screwdriver, and 9 screws.1 Improvements in hand fractures. -

A Study on Management of Comminuted Colles Fracture by Closed Reduction and Ulnocarpal Stabilisation with 2 K-Wires
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 14, Issue 4 Ver. IV (Apr. 2015), PP 45-51 www.iosrjournals.org A Study on Management of Comminuted Colles Fracture by Closed Reduction and Ulnocarpal Stabilisation with 2 K-Wires Dr. Addepalli Srinivasa Rao, M.S, M.ch (Ortho), Dr. K.N.Sandeep M.S(Ortho) Department of Orthopaedics, Siddhartha Medical College, Vijayawada, Andhrapradesh ,India 520008 Abstract Background – In comminuted Colles fractures treated by conventional method , malunion during healing due to progressive radial collapse is a common complication. Many modalities of treatment have been described with their merits and demerits. Ulnocarpal stabilization is an effective method to prevent radial collapse and hence this study. Materials And Methods – A prospective study of 100 patients of comminuted Colles fracture between 20-70 years age irrespective of sex treated by closed reduction and percutaneous stabilization of ulnocarpal articulation and above elbow POP cast for 6weeks has been presented. Patients were evaluated at 1 year follow up and functionally by Sarmiento’s modification of Lindstrom criteria and Gartland and Werley’s criteria. Results – Excellent to good results in 92%,fair in 4% and poor in 4% of total cases. Complications observed were malunion (n=6), pin tract infection (n=7), pullout of k-wire (n=5), sudeck’s osteodystrophy (n=7), residual pain (n=4),reduced grip strength (n=8) . Conclusion – Percutaneous pinnng by ulnocarpal stabilization is minimally invasive, yet an effective method to maintain the reduction and stability of distal radioulnar joint and radial collapse during healing ,even when the fracture is grossly comminuted ,intraarticular or unstable . -

Functional Outcome of Orthogonal Plating in Treatment of Distal
International Journal of Orthopaedics Sciences 2018; 4(2): 78-83 ISSN: 2395-1958 IJOS 2018; 4(2): 78-83 © 2018 IJOS Functional outcome of orthogonal plating in treatment www.orthopaper.com Received: 01-02-2018 of distal humerus fracture Accepted: 05-03-2018 Rajeev Kelkar Rajeev Kelkar and Deepak Singh Rajput Assistant professor, Department of Orthopaedics, MGM Medical College & M.Y. Hospital, Indore, DOI: https://doi.org/10.22271/ortho.2018.v4.i2e.41 M.P, India Abstract Deepak Singh Rajput Background: The study was conducted to evaluate the outcome of orthogonal plating system in distal Resident, Department of humerus intraarticular fracture. Orthopaedics, M.Y. Hospital, Material and Methods: 15 patients of age between 18 to 60 yrs with fracture distal end of humerus with Indore, M.P, India intraarticular extension were evaluated, with the mean age group of 37 to find the functional outcome of Orthogonal plating using olecranon osteotomy approach. Results: The mean union time was 9.53 weeks. The arc of flexion was 99.66o. Average mayo elbow performance score (MEPS) was 83. There were 2 case of infection. One case of implant failure noted secondary to infection leading to implant removal. Discussion and Conclusion: The following results were assessed: operating time, arc of flexion and extension, time to fracture union, functional recovery, and complications. By using proper principles of stable fracture fixation and appropriate exposure in intraarticular fracture (with transolecrenon approach) a good reduction can be achieved which leads to good union, which helps in early mobilization and restoring elbow functions with early intensive physiotherapy. Keywords: Functional outcome, plating, treatment, humerus fracture Introduction Distal Humerus fracture are relatively uncommon and comprise approximately 2-6 % of all fractures [2, 3, 21]. -
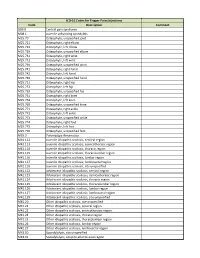
ICD-10 Codes for Trigger Point Injections
ICD-10 Codes for Trigger Point Injections Code Description Comment G89.0 Central pain syndrome M08.1 Juvenile ankylosing spondylitis M25.70 Osteophyte, unspecified joint M25.721 Osteophyte, right elbow M25.722 Osteophyte, left elbow M25.729 Osteophyte, unspecified elbow M25.731 Osteophyte, right wrist M25.732 Osteophyte, left wrist M25.739 Osteophyte, unspecified wrist M25.741 Osteophyte, right hand M25.742 Osteophyte, left hand M25.749 Osteophyte, unspecified hand M25.751 Osteophyte, right hip M25.752 Osteophyte, left hip M25.759 Osteophyte, unspecified hip M25.761 Osteophyte, right knee M25.762 Osteophyte, left knee M25.769 Osteophyte, unspecified knee M25.771 Osteophyte, right ankle M25.772 Osteophyte, left ankle M25.773 Osteophyte, unspecified ankle M25.774 Osteophyte, right foot M25.775 Osteophyte, left foot M25.776 Osteophyte, unspecified foot M35.3 Polymyalgia rheumatica M41.112 Juvenile idiopathic scoliosis, cervical region M41.113 Juvenile idiopathic scoliosis, cervicothoracic region M41.114 Juvenile idiopathic scoliosis, thoracic region M41.115 Juvenile idiopathic scoliosis, thoracolumbar region M41.116 Juvenile idiopathic scoliosis, lumbar region M41.117 Juvenile idiopathic scoliosis, lumbosacral region M41.119 Juvenile idiopathic scoliosis, site unspecified M41.122 Adolescent idiopathic scoliosis, cervical region M41.123 Adolescent idiopathic scoliosis, cervicothoracic region M41.124 Adolescent idiopathic scoliosis, thoracic region M41.125 Adolescent idiopathic scoliosis, thoracolumbar region M41.126 Adolescent idiopathic -

Guideline Treatment of Distal Radius Fractures in Adults
Treatment of distal radius fractures in adults - Norwegian Orthopaedic Association - The Norwegian Medical Association Treatment of distal radius fractures in adults Main Editor Publishing Info Main editor- and author: Hebe Désirée Kvernmo; Co- v2.6 published on authors: Leiv M. Hove, Katrine Bjørnebek Frønsdal, Ingrid 10.08.2015 Harboe, Adalsteinn Odinsson, Yngvar Krukhaug Norwegian Orthopaedic Association - The Norwegian Medical Association 1 of 111 Treatment of distal radius fractures in adults - Norwegian Orthopaedic Association - The Norwegian Medical Association Treatment of distal radius fractures in adults Contact Info Hebe Désirée Kvernmo Department of Orthopaedic-, Plastic- and Hand Surgery, University Hospital of North Norway, N- 9038 Tromsø [email protected] +47 48071311 Language en Creation Date 27.07.2015 Last Edited 27.07.2015 Disclaimer In accordance with new international standards for reliable guidelines and "Guidance on evidence-based medical guidelines" issued by the Norwegian Directorate of Health's, clinical guidelines should include a systematic review of available documentation and a balanced assessment of the benefits and harms of existing treatment options. Clinical guidelines set a standard for assessment, treatment and follow-up of patients or diagnosis groups, and serve as an aid to healthcare personnel in the decision-making in their everyday clinical practice. Professional guidelines are instruments, which purpose is to prevent undesired variation in treatment quality between patients or patient groups. -

Comparison Between Surgical and Conservative Treatment for Distal Radius Fractures in Patients Over 65 Years
Journal of Functional Morphology and Kinesiology Article Comparison between Surgical and Conservative Treatment for Distal Radius Fractures in Patients over 65 Years Gianluca Testa , Andrea Vescio , Paola Di Masi, Giulio Bruno, Giuseppe Sessa and Vito Pavone * Department of General Surgery and Medical Surgical Specialties—Section of Orthopaedic and Traumatology, AOU Policlinico—Vittorio Emanuele, University of Catania, 95123 Catania, Italy; [email protected] (G.T.); [email protected] (A.V.); [email protected] (P.D.M.); [email protected] (G.B.); [email protected] (G.S.) * Correspondence: [email protected]; Tel.: +30-095-378-2273 Received: 12 April 2019; Accepted: 15 May 2019; Published: 17 May 2019 Abstract: Background: Fractures of the distal radius (DRF) are the most common orthopedic injuries, representing one of the typical fractures indicating underlying osteoporosis. The aim of the study was to compare conservative and surgical treatment, analyzing quality of life and clinical outcome in an over 65 years old population. Methods: Ninety one patients were divided into two groups: the ORIF group (39 patients) underwent surgery, and the conservative group (52 patients) was treated conservatively. The clinical and functional outcomes of all patients were evaluated using Short Form 36 (SF36), Modified Mayo Wrist Score (MMWS), Disability of the Arm Shoulder Hand (DASH), and Visual Analogue Scale (VAS). Range of motion at the joint was measured and compared with the contralateral healthy wrist. Results: No significant difference was found between the overall SF36 score, DASH score, MMWS, and VAS results. Role limitation was significantly better in the surgical group (p < 0.05), and complication incidence was significantly higher (p < 0.05) in the conservative group. -

Splinting, Casting, Wrapping and Taping Faculty Disclosure
11/2/2016 Faculty Disclosure I have nothing to disclose. Splinting, Casting, Wrapping and Taping Wade M. Rankin, DO, CAQSM Assistant Professor University of Kentucky College of Medicine, Department of Family and Community Medicine Educational Need/Practice Gap Objectives Gap = Lack of fracture knowledge and ability Upon completion of this educational activity, to manage some outpatient fractures in your you will be able to: outpatient clinic • Outline common fractures seen in outpatient family medicine clinics. • Discuss strategies for fracture management Need = Ability to manage common and fracture care for common outpatient outpatient non-operative fracture care fractures. • Review common indications for taping and wrapping in outpatient settings. Expected Outcome Active Learning -Comfort with common outpatient fractures At the end of this session will discuss certain and ability to manage certain fractures cases and have some audience response -Comfort with splinting, taping, wrapping questions to gauge learning. -Comfort with pre-fabricated splints and common applications 1 11/2/2016 Most Common Fractures? Most Common • Depends on the source: – Clavicle –Arm –Ankle – Wrist – Hip –Foot – Toes –Hand – Finger –Nose Depends on the age: Depends on Age • Common Fractures in Children – 1. Distal radius fractures – 2. Phalanges of the hands – 3. Carpal-metacarpal region – 4. Clavicle Incidence Pediatric Fractures 2 11/2/2016 Types of Pediatric Fractures Salter-Harris Fracture Types Salter-Harris Fracture Types Salter-Harris Fracture I • S- -

Arthroscopic Reduction and Internal Fixation of Distal Radius Fractures
SURGICAL TECHNIQUE Technical Tips for (Dry) Arthroscopic Reduction and Internal Fixation of Distal Radius Fractures Francisco del Piñal, MD, Dr Med Contrary to general belief, arthroscopic assisted reduction in distal radius fractures can be done in an expeditious manner and with minimal consumption of operating room resources. This article presents the steps for a pleasant arthroscopic experience in detail. The technique proposed combines the benefits of rigid fixation with volar locking plates (for the extra- articular component) and arthroscopic control of the reduction (for the articular component). It is important that the operation be carried out using the dry arthroscopic technique. However, arthroscopy is just an addition to conventional methods. Thorough knowledge of Surgical Technique and facility with classic techniques of distal radius fracture treatment is essential for a good result. (J Hand Surg 2011;36A:1694–1705. Copyright © 2011 by the American Society for Surgery of the Hand. All rights reserved.) Key words Wrist arthroscopy, distal radius fracture, intra-articular fracture, volar locking plate. ESTORING THE JOINT ANATOMY is the main goal running out through the incisions and portals obliges us after an articular distal radius fracture, and to combine the less effective Kirschner-wire and exter- fluoroscopy has some inherent inaccuracy.1–4 nal fixation methods,2,5,6,8–10 limiting the use of the R 11,12 On the other hand, the arthroscope allows us to see inside more stable volar-locking plate when arthroscopy the joint with light and magnification. Nevertheless, de- is used. 2,5,6 spite well-performed comparative published studies Arthroscopy without distending the joint with water that support the use of the arthroscope when dealing with (so-called “dry” arthroscopy)13 has many advantages articular distal radius fractures, there is general resistance over the wet (classic) technique but is practiced by few 7 to use the arthroscope. -

Table of Compensation for Injury 1/2015 Valid from 01.04.2015
Table of Compensation for Injury 1/2015 Valid from 01.04.2015 Percentage of Injury compensation 1. CRANIAL INJURIES 1.1 Cranial bone fractures: a) fracture of cranial dome 10 b) fracture of cranial base 15 c) fracture of cranial dome and base 20 1.2 Intracranial haematomas: a) epidural 10 b) subdural, intracerebral 15 1.3 Brain injuries: a) brain concussion 1 b) brain contusion 10 1.4 Injuries to brain, spinal cord and peripheral nervous system: a) spinal cord contusion 6 b) traumatic epilepsy 15 1.5 Traumatic plexitis 10 2. SOFT TISSUES 2.1 Soft tissue burns of II or III degree: a) 1–2% of body surface 3 b) 3–4% of body surface 7 c) 5–6% of body surface 10 d) 7–8% of body surface 15 e) 9–10% of body surface 20 f) 11–20% of body surface 25 g) over 20% of body surface 35 The extent of injury must be established and recorded by a physician immediately after the occurrence of accident. 2.2 Injuries to soft tissues (bites, loose skin tears, wounds, etc.): 1.1.1 at least 2 cm long, requiring stitching: a) In the face and neck area 3 b) In the body area 1 1.1.2 over 10 cm long, requiring stitching 5 1.2 Intermuscular abscess resulting from injury, requiring surgical intervention. 2 Determined 1 month after injury on the basis of a medical certificate. Seesam Insurance AS. Table of Compensation for Injury 1/2015 Percentage of Injury compensation 3. SIGHT ORGANS (extent of injury will be determined 3 months after the insurance incident on the basis of a medical certificate issued during follow-up) 3.1 Functional disorder of lacrimalducts: a) functional disorder of lacrimal duct due to scarring 5 b) traumatic dacryocystitis 15 3.2 After eye injury: a) conjunctivitis, 3 b) keratitis, iridocyclitis 5 c) iris defect, lens luxation, trichiasis, inversion of eyelid 10 3.3 Wounds penetrating eye membrane, burns (corrosion) of II–III degree, hemophthalmus without 5 any degradation of visual acuity 3.4 Orbit fracture 10 4.