Transmissibility of Acute Haemorrhagic Conjunctivitis in Small-Scale
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study on the Evolution of Spatial Forms of Rural Settlements in Xiangjiang River Basin
2019 International Conference on Informatics, Control and Robotics (ICICR 2019) ISBN: 978-1-60595-633-6 Study on the Evolution of Spatial Forms of Rural Settlements in Xiangjiang River Basin Fang LI and Shao-yao HE* School of Architecture, Hunan University, China *Corresponding author Keywords: Xiangjiang River Basin, Rural settlement, Space Form, Evolution. Abstract. The spatial pattern of rural settlements in the Xiangjiang River Basin has changed greatly in both boundary and internal spatial forms. The spatial form of rural settlements has expanded or abruptly evolved, and the internal pattern has changed to varying degrees. This is the natural environment at that time. The economic environment and the social environment are closely related. This paper summarizes the rural settlements and takes the Xiangjiang River Basin as an example. Through the literature analysis and case analysis method, the general situation of the Xiangjiang River Basin and the spatial form of rural settlements are studied. At the same time, the evolution of the settlement form of the Xiangjiang River Basin is quantified. The analysis shows the diversified settlement evolution mechanism. Finally, it discusses the future of the rural settlements in the Xiangjiang River Basin and hopes to promote the sustainable development of the village. Preface Compared with foreign countries, the domestic eye corners of rural settlements and their spatial forms are relatively late. Since the introduction of the relationship between rural settlements and the natural environment in China in the 1930s, Lin Chao studied the classification of rural settlements in 1938 and pointed out that land is closely related to the development of rural settlements. -

The Urban Flood Control Project in the Mountainous Area in Hunan Province Loaned by the Asian Development Bank
The Urban Flood Control Project in the Mountainous Area in Hunan Province Loaned by the Asian Development Bank The External Resettlement Monitoring & Assessment Report (Lengshuijiang City, Lianyuan City, Shuangfeng County, Shaoyang City, Shaodong County, Longhui County, Jiangyong County, Xintian County, Jianghua County, Qiyang County, Ningyuan County, Chenzhou City, Zhuzhou City, Liling City, Zhuzhou County and Youxian County) No.1, 2008 Total No. 1 Hunan Water & Electricity Consulting Corporation (HWECC) September, 2008 Approved by: Wang Hengyang Reviewed by: Long Xiachu Prepared by: Long Xiachu, Wei Riwen 2 Contents 1. Introduction 2. Project Outline 2.1 Project Outline 2.2 Resettlement Outline 3. Establishment and Operation of Resettlement Organizations 3.1 Organization Arrangement 3.2 Organization Operation 4. Project Implementation Progress 4.1 Jiangyong County 4.2 Chenzhou City 5. Resettlement Implementation Progress 5.1 Resettlement Implementation Schedule 5.2 Resettlement Policy and Compensation Standards 5.3 Progress of Land Acquisition 5.4 Progress of Resettlement Arrangement 5.5 Removal Progress of Enterprises and Institutions 5.6 Progress of Resettlement Area Construction 5.7 Arrival and Payment of the Resettlement Fund 6. Psychology and Complaint of the Resettled People 6.1 Complaint Channel 6.2 Complaint Procedures 7. Public Participation, Consultation and Information Publicizing 7.1 Jiangyong County 7.2 Chenzhou City 8. Existed Problems and Suggestions 3 1. Introduction The Urban Flood Control Project in the Mountainous -

Report on Domestic Animal Genetic Resources in China
Country Report for the Preparation of the First Report on the State of the World’s Animal Genetic Resources Report on Domestic Animal Genetic Resources in China June 2003 Beijing CONTENTS Executive Summary Biological diversity is the basis for the existence and development of human society and has aroused the increasing great attention of international society. In June 1992, more than 150 countries including China had jointly signed the "Pact of Biological Diversity". Domestic animal genetic resources are an important component of biological diversity, precious resources formed through long-term evolution, and also the closest and most direct part of relation with human beings. Therefore, in order to realize a sustainable, stable and high-efficient animal production, it is of great significance to meet even higher demand for animal and poultry product varieties and quality by human society, strengthen conservation, and effective, rational and sustainable utilization of animal and poultry genetic resources. The "Report on Domestic Animal Genetic Resources in China" (hereinafter referred to as the "Report") was compiled in accordance with the requirements of the "World Status of Animal Genetic Resource " compiled by the FAO. The Ministry of Agriculture" (MOA) has attached great importance to the compilation of the Report, organized nearly 20 experts from administrative, technical extension, research institutes and universities to participate in the compilation team. In 1999, the first meeting of the compilation staff members had been held in the National Animal Husbandry and Veterinary Service, discussed on the compilation outline and division of labor in the Report compilation, and smoothly fulfilled the tasks to each of the compilers. -

Corporate Social Responsibility White Paper
2020 CEIBS CORPORATE SOCIAL RESPONSIBILITY WHITE PAPER FOREWORD The Covid-19 pandemic has brought mounting research teams, as well as alumni associations and com- uncertainties and complexities to the world economy. Our panies. The professors obtained the research presented globalized society faces the challenge of bringing the in the paper through the employment of detailed CSR virus under control while minimizing its impact on the parameters focused on business leaders, employee economy. Economic difficulties substantially heighten the behavior and their relationship to the external environ- urgency for a more equitable and sustainable society. ment. This granular and nuanced form of research is a powerful tool for guiding the healthy development of CSR. At the same time, there is an ever-pressing need to enrich and expand the CSR framework in the context of The five CEIBS alumni companies featured in the social and economic development. CEIBS has incorporat- white paper offer exceptional examples of aligning busi- ed CSR programs into teaching, research, and student/ ness practices with social needs. Their learning-based alumni activities since its inception. The international busi- future-proof business innovations are a powerful demon- ness school jointly founded by the Chinese government stration of how best to bring CSR to the forefront of busi- and the European Union has accelerated knowledge ness activities. These five firms all received the CSR creation and dissemination during the pandemic to sup- Award in April 2019 at the second CEIBS Alumni Corpo- port economic stability and business development. The rate Social Responsibility Award, organized by the CEIBS institution has also served as a key communication chan- Alumni Association. -

Composition of Garnet from the Xianghualing Skarn Sn Deposit, South China: Its Petrogenetic Significance and Exploration Potential
minerals Article Composition of Garnet from the Xianghualing Skarn Sn Deposit, South China: Its Petrogenetic Significance and Exploration Potential Fan Yu 1, Qihai Shu 1,2,* , Xudong Niu 1, Kai Xing 1,3, Linlong Li 1,4, David R. Lentz 3 , Qingwen Zeng 1 and Wenjie Yang 1 1 State Key Laboratory of Geological Processes and Mineral Resources, School of Earth Sciences and Resources, China University of Geosciences, Beijing 100083, China; [email protected] (F.Y.); [email protected] (X.N.); [email protected] (K.X.); [email protected] (L.L.); [email protected] (Q.Z.); [email protected] (W.Y.) 2 Key Laboratory of Deep-Earth Dynamics of Ministry of Natural Resources, Chinese Academy of Geological Sciences, Beijing 100037, China 3 Department of Earth Sciences, University of New Brunswick, Fredericton, NB E3B 5A3, Canada; [email protected] 4 School of Earth and Space Sciences, Peking University, Beijing 100871, China * Correspondence: [email protected]; Tel.: +86-10-82322750 Received: 11 April 2020; Accepted: 15 May 2020; Published: 18 May 2020 Abstract: The Xianghualing skarn Sn deposit in the southwestern part of the southern Hunan Metallogenic Belt is a large Sn deposit in the Nanling area. In this paper, the garnet has been analyzed by laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) to obtain the concentrations of the major and trace elements. The results reveal that the garnets from the Xianghualing deposit mainly belong to andradite-grossular (grandite) solid solution and are typically richer in Al than in Fe. They show enrichment in heavy rare earth elements (HREEs) and notably lower light rare earth elements (LREEs), and commonly negative Eu anomalies, indicative of a relatively reduced formation environment. -

World Bank Document
Hunan Integrated Management of Agricultural Land Pollution (P153115) Procurement Plan I. General Public Disclosure Authorized 1. Bank’s approval Date of the procurement Plan [original: June 27, 2017; 1st Revision: August 17, 2017; 2nd Revision: June 8, 2018; 3rd Revision: Aug. 10, 2018] 2. Date of General Procurement Notice: August 16, 2017 3. Period covered by this procurement plan: August 2017 to February 2019 II. Goods, Works, non-consulting services and Community participation in procurement under Component 1. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: Procurement Method Prior Review Threshold Procurement Method Threshold US$ US$ Public Disclosure Authorized ICB and LIB (Goods and Non- Greater than or equal to US$ 1. All Consulting Services ) 10 million NCB (Goods and Non-Consulting Greater than or equal to US$ Greater than or equal to 2. Services ) 0.5 million 2 million Greater than or equal to US$ 3. ICB (Works) All 40 million Greater than or equal to US$ Greater than or equal to 4. NCB (Works) 0.5 million 10 million Community participation in to be specified in the 5 No Threshold procurement operation manual Public Disclosure Authorized 2. Prequalification. Bidders for _Not applicable_ shall be prequalified in accordance with the provisions of paragraphs 2.9 and 2.10 of the Guidelines. 3. Proposed Procedures for CDD Components (as per paragraph. 3.17 of the Guidelines: Detailed procedures for community participation are specified in operation manual. 4. Reference to (if any) Project Operational/Procurement Manual: Project Implementation Manual for World Bank Loan Project P153115 has been prepared by Hunan PPMO. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

World Bank Document
Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Attached Figure 1-1 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Baojing County 1 Public Disclosure Authorized Attached Figure 1-2 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Jishou City 2 Attached Figure 1-3 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Yongshun County 3 Diagrammatic Sketch of Location of Demonstration Area of Integrated Management of Agricultural Land Pollution Project with World Bank Loan in Huayuan County Attached Figure 1-4 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Huayuan County 4 Attached Figure 1-5 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Cili County 5 Attached Figure 1-6 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Huayuan County 6 Attached Figure 1-7 Diagrammatic Sketch of Location of Demonstration Area and Distribution of Environmental Sensitive Points and Scope of Evaluation in Anhua County 7 Schematic Diagram for Demonstration Areas of Integrated Management of Agricultural Land Pollution in Hengnan County -

Cui Chenzhou, Zhao Yongheng, CAO Zihuang, Kubánek Petr
Curriculum Vitae Chenzhou CUI National Astronomical Observatories, Chinese Academy of Sciences (NAOC) 20A Datun Road, Chaoyang District Beijing 100012, China Tel: 86-10-64872500 Fax: 86-10-64878240 Email: [email protected] http://www.lamost.org/~cb/ Personal Information: Gender: Male Date of Birth: January 27, 1976 (Chinese Lunar Calendar) Family Status: Married, one child Nationality: P. R. of China Current Position: Chief Information Officer, Professor, NAOC, 2006.1- Education: Ph.D. in Astrophysics, NAOC, Beijing, 2003.6 Concentrations: Virtual Observatory, Grid Technology, Web Services, Scientific Databases Thesis: System Design of Chinese Virtual Observatory Advisor: Prof. Yongheng ZHAO M.Sc. in Astrophysics, Beijing Astronomical Observatory, Beijing, 2000.6 Concentrations: Astronmical Databasese, Galactic Abundances Thesis: Setup of SAGE Astronomical Database and Statistical Analyses of Galactic Abundances Advisor: Prof. Gang ZHAO B.S.E. in Mechanical Engineering, Hebei University of Technology, Tianjin, 1997.6 Research Interests: Astro-Informatics, Virtual Observatory, Grid Technology, Astronomical Databases, Astronomical Data Center, Data Processing and Analysis, Galactic Structure and Evolution, Stellar Abundance Work Experiences: Professor, NAOC, 2013.2 - Associated Professor, NAOC, 2006.1- 2013.1 Lecturer, NAOC, 2003.6-2005.12 Visiting Experiences: Visiting Scientist, Johns Hopkins University, USA, 2007.11.26-2007.12.24 Lecture, International School for Young Astronomers 2007, International Astronomical Union (IAU), Malaysia, -

Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China Wei Wang,1 Jean Artois,1 Xiling Wang, Adam J
RESEARCH Effectiveness of Live Poultry Market Interventions on Human Infection with Avian Influenza A(H7N9) Virus, China Wei Wang,1 Jean Artois,1 Xiling Wang, Adam J. Kucharski, Yao Pei, Xin Tong, Victor Virlogeux, Peng Wu, Benjamin J. Cowling, Marius Gilbert,2 Hongjie Yu2 September 2017) as of March 2, 2018 (2). Compared Various interventions for live poultry markets (LPMs) have with the previous 4 epidemic waves, the 2016–17 fifth emerged to control outbreaks of avian influenza A(H7N9) virus in mainland China since March 2013. We assessed wave raised global concerns because of several char- the effectiveness of various LPM interventions in reduc- acteristics. First, a surge in laboratory-confirmed cas- ing transmission of H7N9 virus across 5 annual waves es of H7N9 virus infection was observed in wave 5, during 2013–2018, especially in the final wave. With along with some clusters of limited human-to-human the exception of waves 1 and 4, various LPM interven- transmission (3,4). Second, a highly pathogenic avi- tions reduced daily incidence rates significantly across an influenza H7N9 virus infection was confirmed in waves. Four LPM interventions led to a mean reduction Guangdong Province and has caused further human of 34%–98% in the daily number of infections in wave 5. infections in 3 provinces (5,6). The genetic divergence Of these, permanent closure provided the most effective of H7N9 virus, its geographic spread (7), and a much reduction in human infection with H7N9 virus, followed longer epidemic duration raised concerns about an by long-period, short-period, and recursive closures in enhanced potential pandemic threat in 2016–17. -
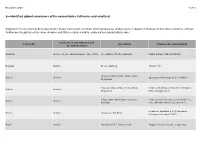
2—Identified Global Consumers of Tin Concentrates (Refineries and Smelters)
November 2020 1 of 12 2—Identified global consumers of tin concentrates (refineries and smelters) Explanation: This list contains global facilities known to be able to process tin concentrate. Additional small-scale smelters may be in operation in Indonesia, but their status could not be confirmed. Facilities were thought to be active unless otherwise noted. Data records are sorted by country and then alphabetically by owner. FACILITY TYPE AND STATUS COUNTRY LOCATION OPERATOR / OWNERSHIP (IF APPLICABLE) Australia Smelter (on care and maintenance since 2012) Greenbushes, Western Australia Global Advanced Metals Pty Ltd. Belgium Smelter Beerse, Antwerp Aurubis AG Huajara Industrial Park, Oruro, Oruro Bolivia Smelter Operaciones Metalúrgicas S.A. (OMSA) Department Vinto smelting complex, Vinto, Oruro Empresa Metalúrgica Vinto S.A. (Compania Bolivia Smelter Department Minera Colquira S.A.) Coopersanta, Bom Futuro, Ariquemes, Cooperativa de Garimperiros de Santa Cruz Brazil Smelter Rondônia Ltda. [Meridian Mineração Jaburi S.A.] Estanho de Rondônia S.A. [Companhia Brazil Smelter Ariquemes, Rondônia Siderúrgica Nacional (CSN)] Brazil Smelter São João del Rei, Minas Gerais Magnu's Minerais Metais e Ligas Ltda. November 2020 2 of 12 2—Identified global consumers of tin concentrates (refineries and smelters) Explanation: This list contains global facilities known to be able to process tin concentrate. Additional small-scale smelters may be in operation in Indonesia, but their status could not be confirmed. Facilities were thought to be active unless otherwise noted. Data records are sorted by country and then alphabetically by owner. FACILITY TYPE AND STATUS COUNTRY LOCATION OPERATOR / OWNERSHIP (IF APPLICABLE) Brazil Smelter Ariquemes, Rondônia Melt Metais e Ligas S.A. -

Spike-Specific Circulating T Follicular Helper Cell and Cross-Neutralizing Antibody Responses in COVID-19-Convalescent Individuals
LETTERS https://doi.org/10.1038/s41564-020-00824-5 Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in COVID-19-convalescent individuals Jian Zhang 1,15, Qian Wu1,15, Ziyan Liu1,15, Qijie Wang2,15, Jiajing Wu3,4,15, Yabin Hu1,15, Tingting Bai5, Ting Xie2, Mincheng Huang2, Tiantian Wu6, Danhong Peng2, Weijin Huang 3, Kun Jin1, Ling Niu1, Wangyuan Guo1, Dixian Luo1, Dongzhu Lei1, Zhijian Wu1, Guicheng Li1, Renbin Huang1, Yingbiao Lin1, Xiangping Xie2, Shuangyan He2, Yunfan Deng7, Jianghua Liu8, Weilang Li9, Zhongyi Lu10, Haifu Chen11, Ting Zeng2, Qingting Luo12, Yi-Ping Li 6 ✉ , Youchun Wang 3 ✉ , Wenpei Liu 1,5,13 ✉ and Xiaowang Qu 1,14 ✉ Coronavirus disease 2019 (COVID-19) is caused by infec- individuals and facilitate the treatment and vaccine develop- tion with severe acute respiratory syndrome coronavirus 2 ment for SARS-CoV-2 infection. (SARS-CoV-2)1–3 and individuals with COVID-19 have symp- To investigate the antibody response after recovery from coro- toms that can be asymptomatic, mild, moderate or severe4,5. In navirus disease 2019 (COVID-19), 67 convalescent individuals the early phase of infection, T- and B-cell counts are substan- were recruited for this study, and blood was drawn on day 28 after tially decreased6,7; however, IgM8–11 and IgG12–14 are detectable discharge. The baseline clinical characteristics and laboratory find- within 14 d after symptom onset. In COVID-19-convalescent ings on admission were retrospectively analysed (Extended Data individuals, spike-specific neutralizing antibodies are vari- Figs. 1 and 2). The binding and avidity of an antibody to a specific able3,15,16.