The First Intracellular Loop Is Essential for the Catalytic Cycle of the Human
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coexpression of ATP-Binding Cassette Proteins ABCG5 and ABCG8 Permits Their Transport to the Apical Surface
Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface Gregory A. Graf, … , Jonathan C. Cohen, Helen H. Hobbs J Clin Invest. 2002;110(5):659-669. https://doi.org/10.1172/JCI16000. Article Cardiology Mutations in either ATP-binding cassette (ABC) G5 or ABCG8 cause sitosterolemia, an autosomal recessive disorder of sterol trafficking. To determine the site of action of ABCG5 and ABCG8, we expressed recombinant, epitope-tagged mouse ABCG5 and ABCG8 in cultured cells. Both ABCG5 and ABCG8 underwent N-linked glycosylation. When either protein was expressed individually in cells, the N-linked sugars remained sensitive to Endoglycosidase H (Endo H). When ABCG5 and ABCG8 were coexpressed, the attached sugars were Endo H–resistant and neuraminidase-sensitive, indicating that the proteins were transported to the trans-Golgi complex. The mature, glycosylated forms of ABCG5 and ABCG8 coimmunoprecipitated, consistent with heterodimerization of these two proteins. The Endo H–sensitive forms of ABCG5 and ABCG8 were confined to the endoplasmic reticulum (ER), whereas the mature forms were present in non-ER fractions in cultured hepatocytes. Immunoelectron microscopy revealed ABCG5 and ABCG8 on the plasma membrane of these cells. In polarized WIF-B cells, recombinant ABCG5 localized to the apical (canalicular) membrane when coexpressed with ABCG8, but not when expressed alone. To our knowledge this is the first direct demonstration that trafficking of an ABC half-transporter to the cell surface requires the presence of its dimerization partner. Find the latest version: https://jci.me/16000/pdf Coexpression of ATP-binding cassette See the related Commentary beginning on page 605. -

Clinical Utility Gene Card For: Sitosterolaemia
European Journal of Human Genetics (2017) 25, doi:10.1038/ejhg.2016.187 & 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 1018-4813/17 www.nature.com/ejhg CLINICAL UTILITY GENE CARD Clinical utility gene card for: Sitosterolaemia Amanda J Hooper1,2,3, Damon A Bell1,2, Robert A Hegele4 and John R Burnett*,1,2 European Journal of Human Genetics (2017) 25, doi:10.1038/ejhg.2016.187; published online 28 December 2016 1. DISEASE CHARACTERISTICS Japanese and Indian patients with sitosterolaemia (20% of known cases), 1.1 Name of the disease (synonyms) it is mainly owing to ABCG5 mutations.4 On the basis of allele Sitosterolaemia (phytosterolaemia; Mediterranean stomatocytosis/ frequencies of loss-of-function variants (frameshift, nonsense and macrothrombocytopenia). splicing only; not missense) in the ExAC database, sitosterolaemia has a global prevalence of at least 1 in 2.6 million for ABCG5 and 1 in 1.2 OMIM# of the disease 360 000 for ABCG8; the most common loss-of-function variant 210250. appears to be ABCG8 c.1083G4A (p.(Trp361Ter)) (Exome Aggrega- tion Consortium; http://exac.broadinstitute.org/) 1.3 Name of the analysed genes or DNA/chromosome segments ABCG5 ABCG8 , . 1.9 Diagnostic setting 1.4 OMIM# of the gene(s) 605459, 605460. Yes No. A. (Differential) diagnostics ⊠ □ 1.5 Mutational spectrum B. Predictive testing □ ⊠ □ ⊠ The sitosterolaemia genes ABCG5 (NM_022436.2) and ABCG8 C. Risk assessment in relatives □ ⊠ (NM_022437.2) lie ‘head to head’ on chromosome 2.1–3 They each D. Prenatal contain 13 exons and encode a half-transporter (sterolin-1 and sterolin-2, respectively), with the C-terminus only containing 6 of Comment: Use of genetic testing is essentially limited to confirmatory 3 the usual 12 transmembrane domains of the other ABC transporters. -

The Rat STSL Locus: Characterization, Chromosomal Assignment, And
BMC Cardiovascular Disorders BioMed Central Research article Open Access The rat STSL locus: characterization, chromosomal assignment, and genetic variations in sitosterolemic hypertensive rats Hongwei Yu1, Bhaswati Pandit1, Eric Klett1, Mi-Hye Lee1, Kangmo Lu1, Khalil Helou2,3, Ikuo Ikeda4, Nami Egashira4, Masao Sato4, Richard Klein1, Ashok Batta5, Gerald Salen5 and Shailendra B Patel*1 Address: 1Division of Endocrinology, Diabetes and Medical Genetics, Medical University of South Carolina, STR 541, 114 Doughty Street, Charleston, SC 29403, USA, 2Genetics Branch, Center for Cancer Research, National Cancer, Institute/NIH, Bethesda, Maryland 20892, USA, 3Department of Oncology, Institute of Selected Clinical Sciences, Goteborg University, SE 413 45, Gothenburg, Sweden, 4Laboratory of Nutrition Chemistry, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School Kyushu University, Fukuoka, 812-8581, Japan and 5Research Service and Medical Service, Department of Veterans Affairs Medical Center, East Orange, NJ, USA Email: Hongwei Yu - [email protected]; Bhaswati Pandit - [email protected]; Eric Klett - [email protected]; Mi-Hye Lee - [email protected]; Kangmo Lu - [email protected]; Khalil Helou - [email protected]; Ikuo Ikeda - [email protected]; Nami Egashira - [email protected]; Masao Sato - [email protected]; Richard Klein - [email protected]; Ashok Batta - [email protected]; Gerald Salen - [email protected]; Shailendra B Patel* - [email protected] * Corresponding author Published: 3 June 2003 Received: 24 January 2003 Accepted: 3 June 2003 BMC Cardiovascular Disorders 2003, 3:4 This article is available from: http://www.biomedcentral.com/1471-2261/3/4 © 2003 Yu et al; licensee BioMed Central Ltd. -

Cardiovascular Disease Products
Cardiovascular Disease Products For more information, visit: www.bosterbio.com Cardiovascular Disease Research Cardiovascular disease is the leading cause of death in developed nations. Boster Bio aims to supply researchers with high-quality antibodies and ELISA kits so they can make new discoveries and help save lives. In this catalogue you will find a comprehensive list of high-affinity Boster antibodies and high sensitivity Boster ELISA kits targeted at proteins associated with cardiovascular disease. Boster: The Fastest Growing About Bosterbio Antibody Company In 2015 Boster is an antibody manufacturer founded in 1993 by histologist Steven Xia. Over the past two decades, Boster and its products have been cited in over 20,000 publications and counting. The firm specializes in developing antibodies and ELISA kits that feature high affinity, Boster Bio received the CitaAb award for high specificity at affordable the greatest increase in number of prices. citations during 2015 than any other antibody manufacturer. Table of Contents Boster Cardiovascular Disease Related Antibodies…………..………..... 2 Boster Cardiovascular Disease Related ELISA Kits……………………..…. 9 1 High Affinity Boster Antibodies Boster supplies only the highest quality antibodies. Our high-affinity polyclonal and monoclonal antibodies are thoroughly validated by Western Blotting, Immunohistochemistry and ELISA. This is our comprehensive catalog of our antibody products related to cardiovascular disease, sorted in alphabetical order by target gene name. Catalog No Product Name -
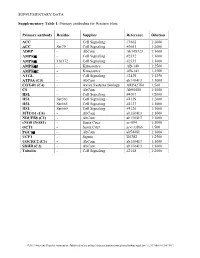
Supplementary Table 1
SUPPLEMENTARY DATA Supplementary Table 1. Primary antibodies for Western blots. Primary antibody Residue Supplier Reference Dilution ACC - Cell Signaling #3662 1:2000 ACC Ser79 Cell Signaling #3661 1:2000 ADRP - AbCam Ab108323 1:1000 AMPKα - Cell Signaling #2532 1:1000 AMPKα Thr172 Cell Signaling #2535 1:1000 AMPKα1 - Kinasource AB-140 1:2500 AMPKα2 - Kinasource AB-141 1:2500 ATGL - Cell Signaling #2439 1:1250 ATP5A (C5) - AbCam ab110413 1:1000 COX4l1 (C4) - Aviva Systems Biology ARP42784 1:500 CS - AbCam Ab96600 1:1000 HSL - Cell Signaling #4107 1:2000 HSL Ser563 Cell Signaling #4139 1:2000 HSL Ser565 Cell Signaling #4137 1:1000 HSL Ser660 Cell Signaling #4126 1:1000 MTCO1 (C4) - AbCam ab110413 1:1000 NDUFB8 (C1) - AbCam ab110413 1:1000 eNOS (NOS3) - Santa Cruz sc-654 1:1000 OCT1 - Santa Cruz sc-133866 1:500 PGC1α - AbCam ab54481 1:1000 UCP1 - Sigma U6382 1:2500 UQCRC2 (C3) - AbCam ab110413 1:1000 SDHB (C2) - AbCam ab110413 1:1000 Tubulin - Cell Signaling #2148 1:2000 ©2013 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0194/-/DC1 SUPPLEMENTARY DATA Supplementary Table 2. Primer sequences for qRT-PCR. Gene Accession number Forward primer Reverse primer Abca1 NM_013454.3 CCCAGAGCAAAAAGCGACTC GGTCATCATCACTTTGGTCCTTG Abcg5 NM_031884 TGTCCTACAGCGTCAGCAACC GGCCACTCTCGATGTACAAGG Abcg8 NM_026180 TCCTGTGAGCTGGGCATCCGA CCCGCAGCCTGAGCTCCCTAT Acaca NM_133360.2 CAGCTGGTGCAGAGGTACCG TCTACTCGCAGGTACTGCCG Acacb NM_133904.2 GCGCCTACTATGAGGCCCAGCA ACAAACTCGGCTGGGGACGC Acly NM_134037.2 -

A Case of Ezetimibe-Effective Hypercholesterolemia with a Novel Heterozygous Variant in ABCG5
2020, 67 (11), 1099-1105 Original A case of ezetimibe-effective hypercholesterolemia with a novel heterozygous variant in ABCG5 Yujiro Nakano1), Chikara Komiya1), Hitomi Shimizu2), 3), Hiroyuki Mishima3), Kumiko Shiba1), Kazutaka Tsujimoto1), Kenji Ikeda1), Kenichi Kashimada4), Sumito Dateki2), Koh-ichiro Yoshiura3), Yoshihiro Ogawa5) and Tetsuya Yamada1) 1) Department of Molecular Endocrinology and Metabolism, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8519, Japan 2) Department of Pediatrics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaski 852-8501, Japan 3) Department of Human Genetics, Graduate School of Biomedical Sciences, Nagasaki University, Nagasaki 852-8501, Japan 4) Department of Pediatrics and Developmental Biology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo 113-8519, Japan 5) Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka 812-8582, Japan Abstract. Sitosterolemia is caused by homozygous or compound heterozygous gene mutations in either ATP-binding cassette subfamily G member 5 (ABCG5) or 8 (ABCG8). Since ABCG5 and ABCG8 play pivotal roles in the excretion of neutral sterols into feces and bile, patients with sitosterolemia present elevated levels of serum plant sterols and in some cases also hypercholesterolemia. A 48-year-old woman was referred to our hospital for hypercholesterolemia. She had been misdiagnosed with familial hypercholesterolemia at the age of 20 and her serum low-density lipoprotein cholesterol (LDL-C) levels had remained about 200–300 mg/dL at the former clinic. Although the treatment of hydroxymethylglutaryl-CoA (HMG-CoA) reductase inhibitors was ineffective, her serum LDL-C levels were normalized by ezetimibe, a cholesterol transporter inhibitor. -

Common Genetic Variations Involved in the Inter-Individual Variability Of
nutrients Review Common Genetic Variations Involved in the Inter-Individual Variability of Circulating Cholesterol Concentrations in Response to Diets: A Narrative Review of Recent Evidence Mohammad M. H. Abdullah 1 , Itzel Vazquez-Vidal 2, David J. Baer 3, James D. House 4 , Peter J. H. Jones 5 and Charles Desmarchelier 6,* 1 Department of Food Science and Nutrition, Kuwait University, Kuwait City 10002, Kuwait; [email protected] 2 Richardson Centre for Functional Foods & Nutraceuticals, University of Manitoba, Winnipeg, MB R3T 6C5, Canada; [email protected] 3 United States Department of Agriculture, Agricultural Research Service, Beltsville, MD 20705, USA; [email protected] 4 Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, MB R3T 2N2, Canada; [email protected] 5 Nutritional Fundamentals for Health, Vaudreuil-Dorion, QC J7V 5V5, Canada; [email protected] 6 Aix Marseille University, INRAE, INSERM, C2VN, 13005 Marseille, France * Correspondence: [email protected] Abstract: The number of nutrigenetic studies dedicated to the identification of single nucleotide Citation: Abdullah, M.M.H.; polymorphisms (SNPs) modulating blood lipid profiles in response to dietary interventions has Vazquez-Vidal, I.; Baer, D.J.; House, increased considerably over the last decade. However, the robustness of the evidence-based sci- J.D.; Jones, P.J.H.; Desmarchelier, C. ence supporting the area remains to be evaluated. The objective of this review was to present Common Genetic Variations Involved recent findings concerning the effects of interactions between SNPs in genes involved in cholesterol in the Inter-Individual Variability of metabolism and transport, and dietary intakes or interventions on circulating cholesterol concen- Circulating Cholesterol trations, which are causally involved in cardiovascular diseases and established biomarkers of Concentrations in Response to Diets: cardiovascular health. -

Nuclear Transcription Factors That Regulate Sterols Thomas Dayspring MD, FACP
Nuclear Transcription Factors that Regulate Sterols Thomas Dayspring MD, FACP Under circumstances of cholesterol deficiency, Sterol Regulatory Element Binding Proteins (SREBPs) via binding to DNA nuclear response elements set off genomic production of proteins and enzymes that induce cholesterol synthesis, such as HMGCoA reductase. Regulating Hepatic Cellular Cholesterol Hepatic Lipogenic Enzymes Sinusoid Acetyl-CoA Hepatocyte HMG-CoA HMG-CoA Reduction mRNA Reductase Endoplasmic Mevalonate SREBP Reticulum Geranyl-PP Farnesyl-PP Negative Feedback Squalene 19 steps including multiple intermediaries Geranlygeranyl-PP including lathosterol DepletedCholesterol Cholesterol Pool Isoprenoids Synthesis Sterol Regulatory Element Binding Protein 2 (SREBP-2) regulates Cholesterol Synthesis Bile Duct Nuclear Transcription Factors that Regulate Sterols Thomas Dayspring MD, FACP SREBs also induce synthesis of LDL receptors which translocate to the surface of the cell and endocytose lipoproteins with apoE or apoB on their surface. These lipoproteins of course deliver sterols in this “indirect reverse cholesterol transport” process. Upregulation of the Niemann Pick C1 Like 1 protein NPC1L1 at the hepatobiliary interface is another source of cholesterol for the hepatocyte. Regulating Hepatic Cellular Cholesterol Hepatocyte LDL receptor synthesis & translocation to cell membrane Endoplasmic Reticulum Decreased cholesterol pool Cholesteryl mRNA ester Nucleus DNA Sterol Regulatory Element Binding Proteins Hepatic Sinusoid sense the low sterols LDL receptor endocytosis of LDL NPC1L1 Niemann Pick C1 Bile particles carrying cholesteryl ester Like 1 Protein Duct Biliary free NPC1L1 facilitates cholesterol movement cholesterol from bile back to hepatocytes Nuclear Transcription Factors that Regulate Sterols Thomas Dayspring MD, FACP Sterol cellular toxicity (crystallization) is prevented primarily by the liver X receptors (LXR) and the Farnesol or Farnesoid X receptors (FXR). -

Supplemental Data
Supplemental Table 1. Panels (utilized in this study) and the genes included in each of them are summarized below. Nephrotic Syndrome (NS)/Focal ACTN4, ANKFY1, ANLN, APOL1, ARHGAP24, ARHGDIA, CD2AP, CDK20, COL4A3, COL4A4, COL4A5, COL4A6, COQ2, COQ6, COQ8B, CRB2, CUBN, Segmental DGKE, DLC1, EMP2, FAT1, GAPVD1, GON7, INF2, ITGA3, ITGB4, ITSN1, ITSN2, KANK1, KANK2, KANK4, KAT2B, KIRREL1, LAGE3, LAMA5, LAMB2, Glomerulosclerosis LMX1B, MAFB, MAGI2, MYH9, MYO1E, NEU1, NFKB2, NPHS1, NPHS2, NUP107, NUP133, NUP160, NUP205, NUP93, OSGEP, PAX2, PDSS2, PLCE1, (FSGS) Panel PTPRO, SCARB2, SGPL1, SMARCAL1, TBC1D8B, TNS2, TP53RK, TPRKB, TRIM8, TRPC6, TTC21B, WDR4, WDR73, WT1, XPO5, YRDC Alport syndrome COL4A3, COL4A4, COL4A5, COL4A6 Panel Autosomal Dominant Polycystic Kidney DNAJB11, GANAB, HNF1B, PKD1, PKD2 Disease (ADPKD) panel Recessive Polycystic Kidney DZIP1L, PKHD1 Disease (ARPKD) panel Hereditary cystic ANKS6, CEP164, CEP290, CEP83, COL4A1, CRB2, DCDC2, DICER1, DNAJB11, DZIP1L, GANAB, GLIS2, HNF1B, IFT172, INVS, IQCB1, JAG1, LRP5, kidney disease MAPKBP1, MUC1, NEK8, NOTCH2, NPHP1, NPHP3, NPHP4, OFD1, PAX2, PKD1, PKD2, PKHD1, RPGRIP1L, SDCCAG8, SEC61A1, TMEM67, TSC1, panel TSC2, TTC21B, UMOD, VHL, WDR19, ZNF423 Nephrotic NPHS1, NPHS2, WT1, PLCE1, LAMB2 Syndrome Autosomal Dominant and Recessive Polycystic Kidney DNAJB11, DZIP1L, GANAB, HNF1B, PKD1, PKD2, PKHD1 Disease (ADPKD and ARPKD) Panel Distal Renal Tubular Acidosis ATP6V0A4, ATP6V1B1, CA2, SLC4A1 Panel Atypical Hemolytic Uremic syndrome C3, CFB, CFH, CFHR1, CFHR3, CFHR5, CFI, DGKE, MCP, THBD -

Cholesterol and Saturated Fat Intake Determine the Effect Of
article September 2006 ⅐ Vol. 8 ⅐ No. 9 Cholesterol and saturated fat intake determine the effect of polymorphisms at ABCG5/ABCG8 genes on lipid levels in children Enrique Viturro, PhD1, Manuel de Oya, PhD, MD1, Miguel A. Lasuncio´n, PhD2, Lydia Gorgojo, PhD, MD3, Jose´ M. Martı´n Moreno, PhD, MD3, Mercedes Benavente, PhD1, Beatriz Cano, PhD1, and Carmen Garces, PhD1 Purpose: Analysis of mutations in genes of the cholesterol metabolic pathway has not completely explained the interindividual variability of blood cholesterol concentrations attributed to gene–nutrient interactions. Thus, we analyzed polymorphisms in the ABCG5 and ABCG8 genes, involved in the regulation of intestinal cholesterol absorption, with special interest in a potential interaction with diet to determine lipid levels. Methods: The polymorphisms ABCG5 C1950G (Gln604Glu) and ABCG8 C1895T (Ala640Val) were determined by polymerase chain reaction and restriction analysis in 1227 healthy school children, aged 6 to 8 years. Results: No significant differences were found in blood lipid levels between subjects with different genotypes of the two analyzed polymorphisms. However, important differences appeared when separating subjects by their different lipid intake. The presence of the ABCG8 C1895T and ABCG5 C1950G polymorphisms was associated with different plasma total cholesterol, low-density lipoprotein cholesterol complex, and apolipoprotein B levels only in low-cholesterol consumers (significantly for the C1895T polymorphism), and among children within the lower tertile of saturated fat intake (significantly for the C1950G polymorphism). Conclusion: Polymorphisms at the half-transporter ABCG5 and ABCG8 genes affect blood cholesterol concentrations in prepubertal children by influencing dietary respon- siveness. This highly significant gene–nutrient interaction could explain the great individual differences in the plasma lipid response to cholesterol and fat intake. -

Progress in Lipid Research Progress in Lipid Research 46 (2007) 297–314 Review Non-Vesicular Sterol Transport in Cells
Progress in Lipid Research Progress in Lipid Research 46 (2007) 297–314 www.elsevier.com/locate/plipres Review Non-vesicular sterol transport in cells William A. Prinz * Laboratory of Cell Biochemistry and Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, US Department of Health and Human Services, Bethesda, MD 20892, USA Abstract Sterols such as cholesterol are important components of cellular membranes. They are not uniformly distributed among organelles and maintaining the proper distribution of sterols is critical for many cellular functions. Both vesicular and non- vesicular pathways move sterols between membranes and into and out of cells. There is growing evidence that a number of non-vesicular transport pathways operate in cells and, in the past few years, a number of proteins have been proposed to facilitate this transfer. Some are soluble sterol transfer proteins that may move sterol between membranes. Others are inte- gral membranes proteins that mediate sterol efflux, uptake from cells, and perhaps intracellular sterol transfer as well. In most cases, the mechanisms and regulation of these proteins remains poorly understood. This review summarizes our cur- rent knowledge of these proteins and how they could contribute to intracellular sterol trafficking and distribution. Published by Elsevier Ltd. Keywords: Cholestrol; Transport; Non-vesicular; Membranes; Lipid transport proteins Contents 1. Introduction ...................................................................... 298 2. Non-vesicular sterol transport pathways .................................................. 299 2.1. Transfer of cholesterol to the outer and inner mitochondrial membranes . .................... 300 2.2. PM to ERC sterol transport . ........................................................ 300 2.3. Sterol movement to and from lipid droplets (LDs). ........................................ 300 2.4. -

Role of Myeloperoxidase Mediated Oxidative Modification and Apolipoprotein Composition in High Density Lipoprotein Function
ROLE OF MYELOPEROXIDASE MEDIATED OXIDATIVE MODIFICATION AND APOLIPOPROTEIN COMPOSITION IN HIGH DENSITY LIPOPROTEIN FUNCTION by ARUNDHATI UNDURTI Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Thesis Advisor: Dr. Stanley L. Hazen Department of Microbiology and Molecular Biology Cell Biology Program CASE WESTERN RESERVE UNIVERSITY August, 2010 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of _____________________________________________________Arundhati Undurti candidate for the ______________________degreePhD *. Alan Levine (signed)_______________________________________________ (chair of the committee) Stanley Hazen ________________________________________________ Jonathan Smith ________________________________________________ Menachem Shoham ________________________________________________ Mark Chance ________________________________________________ ________________________________________________ (date) _______________________06-30-2010 *We also certify that written approval has been obtained for any proprietary material contained therein. For Amma and Nana TABLE OF CONTENTS List of Figures 3 List of Tables 7 Abbreviations 8 Acknowledgements 11 Abstract 13 Chapter I: Introduction Pathogenesis of Atherosclerosis Endothelial Dysfunction 17 Fatty Streak Formation 17 Advanced Lesion Formation 18 Thrombotic Complications 18 Role of Lipoproteins in Atherosclerosis Lipoprotein Classification and Metabolism 19 High Density Lipoprotein 20 Reverse