Cholesterol and Saturated Fat Intake Determine the Effect Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genes in Eyecare Geneseyedoc 3 W.M
Genes in Eyecare geneseyedoc 3 W.M. Lyle and T.D. Williams 15 Mar 04 This information has been gathered from several sources; however, the principal source is V. A. McKusick’s Mendelian Inheritance in Man on CD-ROM. Baltimore, Johns Hopkins University Press, 1998. Other sources include McKusick’s, Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders. Baltimore. Johns Hopkins University Press 1998 (12th edition). http://www.ncbi.nlm.nih.gov/Omim See also S.P.Daiger, L.S. Sullivan, and B.J.F. Rossiter Ret Net http://www.sph.uth.tmc.edu/Retnet disease.htm/. Also E.I. Traboulsi’s, Genetic Diseases of the Eye, New York, Oxford University Press, 1998. And Genetics in Primary Eyecare and Clinical Medicine by M.R. Seashore and R.S.Wappner, Appleton and Lange 1996. M. Ridley’s book Genome published in 2000 by Perennial provides additional information. Ridley estimates that we have 60,000 to 80,000 genes. See also R.M. Henig’s book The Monk in the Garden: The Lost and Found Genius of Gregor Mendel, published by Houghton Mifflin in 2001 which tells about the Father of Genetics. The 3rd edition of F. H. Roy’s book Ocular Syndromes and Systemic Diseases published by Lippincott Williams & Wilkins in 2002 facilitates differential diagnosis. Additional information is provided in D. Pavan-Langston’s Manual of Ocular Diagnosis and Therapy (5th edition) published by Lippincott Williams & Wilkins in 2002. M.A. Foote wrote Basic Human Genetics for Medical Writers in the AMWA Journal 2002;17:7-17. A compilation such as this might suggest that one gene = one disease. -

The Crucial Roles of Apolipoproteins E and C-III in Apob Lipoprotein Metabolism in Normolipidemia and Hypertriglyceridemia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Harvard University - DASH The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Sacks, Frank M. 2015. “The Crucial Roles of Apolipoproteins E and C-III in apoB Lipoprotein Metabolism in Normolipidemia and Hypertriglyceridemia.” Current Opinion in Lipidology 26 (1) (February): 56–63. doi:10.1097/mol.0000000000000146. Published Version doi:10.1097/MOL.0000000000000146 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:30203554 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP HHS Public Access Author manuscript Author Manuscript Author ManuscriptCurr Opin Author Manuscript Lipidol. Author Author Manuscript manuscript; available in PMC 2016 February 01. Published in final edited form as: Curr Opin Lipidol. 2015 February ; 26(1): 56–63. doi:10.1097/MOL.0000000000000146. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia Frank M. Sacks Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts, USA Abstract Purpose of review—To describe the roles of apolipoprotein C-III (apoC-III) and apoE in VLDL and LDL metabolism Recent findings—ApoC-III can block clearance from the circulation of apolipoprotein B (apoB) lipoproteins, whereas apoE mediates their clearance. -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -

Coexpression of ATP-Binding Cassette Proteins ABCG5 and ABCG8 Permits Their Transport to the Apical Surface
Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface Gregory A. Graf, … , Jonathan C. Cohen, Helen H. Hobbs J Clin Invest. 2002;110(5):659-669. https://doi.org/10.1172/JCI16000. Article Cardiology Mutations in either ATP-binding cassette (ABC) G5 or ABCG8 cause sitosterolemia, an autosomal recessive disorder of sterol trafficking. To determine the site of action of ABCG5 and ABCG8, we expressed recombinant, epitope-tagged mouse ABCG5 and ABCG8 in cultured cells. Both ABCG5 and ABCG8 underwent N-linked glycosylation. When either protein was expressed individually in cells, the N-linked sugars remained sensitive to Endoglycosidase H (Endo H). When ABCG5 and ABCG8 were coexpressed, the attached sugars were Endo H–resistant and neuraminidase-sensitive, indicating that the proteins were transported to the trans-Golgi complex. The mature, glycosylated forms of ABCG5 and ABCG8 coimmunoprecipitated, consistent with heterodimerization of these two proteins. The Endo H–sensitive forms of ABCG5 and ABCG8 were confined to the endoplasmic reticulum (ER), whereas the mature forms were present in non-ER fractions in cultured hepatocytes. Immunoelectron microscopy revealed ABCG5 and ABCG8 on the plasma membrane of these cells. In polarized WIF-B cells, recombinant ABCG5 localized to the apical (canalicular) membrane when coexpressed with ABCG8, but not when expressed alone. To our knowledge this is the first direct demonstration that trafficking of an ABC half-transporter to the cell surface requires the presence of its dimerization partner. Find the latest version: https://jci.me/16000/pdf Coexpression of ATP-binding cassette See the related Commentary beginning on page 605. -

'Sitosterolemia—10 Years Observation in Two Sisters'
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 Sitosterolemia—10 years observation in two sisters Veit, Lara ; Allegri Machado, Gabriella ; Bürer, Céline ; Speer, Oliver ; Häberle, Johannes Abstract: Familial hypercholesterolemia due to heterozygous low‐density lipoprotein‐receptor mutations is a common inborn errors of metabolism. Secondary hypercholesterolemia due to a defect in phytosterol metabolism is far less common and may escape diagnosis during the work‐up of patients with dyslipi- demias. Here we report on two sisters with the rare, autosomal recessive condition, sitosterolemia. This disease is caused by mutations in a defective adenosine triphosphate‐binding cassette sterol excretion transporter, leading to highly elevated plant sterol concentrations in tissues and to a wide range of symp- toms. After a delayed diagnosis, treatment with a diet low in plant lipids plus ezetimibe to block the absorption of sterols corrected most of the clinical and biochemical signs of the disease. We followed the two patients for over 10 years and report their initial presentation and long‐term response to treatment. DOI: https://doi.org/10.1002/jmd2.12038 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-182906 Journal Article Accepted Version Originally published at: Veit, Lara; Allegri Machado, Gabriella; Bürer, Céline; Speer, Oliver; Häberle, Johannes (2019). Sitosterolemia— 10 years observation in -

Clinical Utility Gene Card For: Sitosterolaemia
European Journal of Human Genetics (2017) 25, doi:10.1038/ejhg.2016.187 & 2017 Macmillan Publishers Limited, part of Springer Nature. All rights reserved 1018-4813/17 www.nature.com/ejhg CLINICAL UTILITY GENE CARD Clinical utility gene card for: Sitosterolaemia Amanda J Hooper1,2,3, Damon A Bell1,2, Robert A Hegele4 and John R Burnett*,1,2 European Journal of Human Genetics (2017) 25, doi:10.1038/ejhg.2016.187; published online 28 December 2016 1. DISEASE CHARACTERISTICS Japanese and Indian patients with sitosterolaemia (20% of known cases), 1.1 Name of the disease (synonyms) it is mainly owing to ABCG5 mutations.4 On the basis of allele Sitosterolaemia (phytosterolaemia; Mediterranean stomatocytosis/ frequencies of loss-of-function variants (frameshift, nonsense and macrothrombocytopenia). splicing only; not missense) in the ExAC database, sitosterolaemia has a global prevalence of at least 1 in 2.6 million for ABCG5 and 1 in 1.2 OMIM# of the disease 360 000 for ABCG8; the most common loss-of-function variant 210250. appears to be ABCG8 c.1083G4A (p.(Trp361Ter)) (Exome Aggrega- tion Consortium; http://exac.broadinstitute.org/) 1.3 Name of the analysed genes or DNA/chromosome segments ABCG5 ABCG8 , . 1.9 Diagnostic setting 1.4 OMIM# of the gene(s) 605459, 605460. Yes No. A. (Differential) diagnostics ⊠ □ 1.5 Mutational spectrum B. Predictive testing □ ⊠ □ ⊠ The sitosterolaemia genes ABCG5 (NM_022436.2) and ABCG8 C. Risk assessment in relatives □ ⊠ (NM_022437.2) lie ‘head to head’ on chromosome 2.1–3 They each D. Prenatal contain 13 exons and encode a half-transporter (sterolin-1 and sterolin-2, respectively), with the C-terminus only containing 6 of Comment: Use of genetic testing is essentially limited to confirmatory 3 the usual 12 transmembrane domains of the other ABC transporters. -

Comprehensive Test Menu | August 2020
Comprehensive Test Menu | August 2020 Cancer brain tumors Disease/Condition Test Name/Gene(s) TAT Test Code Brain tumors, hereditary BrainTumorNext®*: 29 genes 14-21 days 8847, 8847-R Neurofibromatosis type 2 (NF2) NF2 14-21 days 9024 Schwannomatosis SMARCB1 14-21 days 7180 breast and gynecologic cancer Disease/Condition Test Name/Gene(s) TAT Test Code Ataxia-telangiectasia ATM 14-21 days 9014 Breast cancer, hereditary BRCA1/BRCA2 6-10 days 8838 BRCA Ashkenazi Jewish 3-site mutation panel 6-10 days 5892 BRCAplus®: 8 genes 7-10 days 8836 BRCANext™*: 18 genes 14-21 days 8855, 8855-R BRCANext-Expanded™*: 23 genes 14-21 days 8860, 8860-R CHEK2-related cancer CHEK2 14-21 days 9016 Li-Fraumeni syndrome TP53 14-21 days 2866 Ovarian, breast and uterine cancer, hereditary BRCANext*: 18 genes 14-21 days 8855, 8855-R BRCANext-Expanded*: 23 genes 14-21 days 8860, 8860-R Ovarian cancer, paired tumor/germline TumorNext®-HRD 21-28 days 9811 TumorNext-BRCA 21-28 days 9810 PALB2-associated cancer PALB2 14-21 days 2366 PTEN-related disorders (Cowden syndrome, Proteus PTEN 14-21 days 2106 syndrome, macrocephaly and autism) comprehensive cancer Disease/Condition Test Name/Gene(s) TAT Test Code Breast, ovarian, colorectal, uterine, pancreatic, prostate, CancerNext®*: 36 genes 14-21 days 8824, 8824-R and other cancer CancerNext-Expanded®*: 77 genes 14-21 days 8874, 8874-R CustomNext-Cancer®*: choose up to 91 genes 14-21 days 9510, 9510-R endocrine tumors Disease/Condition Test Name/Gene(s) TAT Test Code Multiple endocrine neoplasia type 1 (MEN1) MEN1 14-21 -

Cardiovascular Disease Products
Cardiovascular Disease Products For more information, visit: www.bosterbio.com Cardiovascular Disease Research Cardiovascular disease is the leading cause of death in developed nations. Boster Bio aims to supply researchers with high-quality antibodies and ELISA kits so they can make new discoveries and help save lives. In this catalogue you will find a comprehensive list of high-affinity Boster antibodies and high sensitivity Boster ELISA kits targeted at proteins associated with cardiovascular disease. Boster: The Fastest Growing About Bosterbio Antibody Company In 2015 Boster is an antibody manufacturer founded in 1993 by histologist Steven Xia. Over the past two decades, Boster and its products have been cited in over 20,000 publications and counting. The firm specializes in developing antibodies and ELISA kits that feature high affinity, Boster Bio received the CitaAb award for high specificity at affordable the greatest increase in number of prices. citations during 2015 than any other antibody manufacturer. Table of Contents Boster Cardiovascular Disease Related Antibodies…………..………..... 2 Boster Cardiovascular Disease Related ELISA Kits……………………..…. 9 1 High Affinity Boster Antibodies Boster supplies only the highest quality antibodies. Our high-affinity polyclonal and monoclonal antibodies are thoroughly validated by Western Blotting, Immunohistochemistry and ELISA. This is our comprehensive catalog of our antibody products related to cardiovascular disease, sorted in alphabetical order by target gene name. Catalog No Product Name -

Apolipoproteins and Their Association with Cardiometabolic Risk
Nutr Hosp. 2015;32(6):2674-2683 ISSN 0212-1611 • CODEN NUHOEQ S.V.R. 318 Original / Síndrome metabólico Apolipoproteins and their association with cardiometabolic risk biomarkers in adolescents Mellina Neyla de Lima Albuquerque1, Alcides da Silva Diniz1 and Ilma Kruze Grande de Arruda1 1Department of Nutrition. Federal University of Pernambuco, Recife, Brazil. Abstract APOLIPOPROTEÍNAS Y SU ASOCIACIÓN CON BIOMARCADORES DE RIESGO Introduction: the apoB/apo A-I ratio has been reported CARDIOMETABÓLICO EN ADOLESCENTES as an important predictor of cardiovascular risk, being superior to lipids, lipoproteins and conventional lipid ra- tios. Resumen Objective: to investigate the association between apo- Introducción: la razón apo B/apo A-I sigue siendo lipoproteins A-I and B, and the apolipoprotein B/apoli- reportada como un predictor importante de riesgo car- poprotein A-I ratio and cardiometabolic risk variables in diovascular, superior a lípidos, lipoproteínas y razones adolescents. lipídicas convencionales. Objetivo: investigar la asocia- Methods: this was a cross-sectional study including ción entre las apolipoproteínas A-I y B y la razón apoli- 104 adolescents of public schools in Recife during the poproteína B/apolipoproteína A-I con variables de riesgo months of March/April, 2013. Sociodemographic, an- cardiometabólico en adolescentes. thropometric, clinical and biochemical variables were Métodos: estudio de corte transversal que incluye a analysed. The apolipoproteins were analysed via Immu- 104 adolescentes de escuelas públicas de -
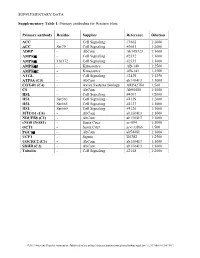
Supplementary Table 1
SUPPLEMENTARY DATA Supplementary Table 1. Primary antibodies for Western blots. Primary antibody Residue Supplier Reference Dilution ACC - Cell Signaling #3662 1:2000 ACC Ser79 Cell Signaling #3661 1:2000 ADRP - AbCam Ab108323 1:1000 AMPKα - Cell Signaling #2532 1:1000 AMPKα Thr172 Cell Signaling #2535 1:1000 AMPKα1 - Kinasource AB-140 1:2500 AMPKα2 - Kinasource AB-141 1:2500 ATGL - Cell Signaling #2439 1:1250 ATP5A (C5) - AbCam ab110413 1:1000 COX4l1 (C4) - Aviva Systems Biology ARP42784 1:500 CS - AbCam Ab96600 1:1000 HSL - Cell Signaling #4107 1:2000 HSL Ser563 Cell Signaling #4139 1:2000 HSL Ser565 Cell Signaling #4137 1:1000 HSL Ser660 Cell Signaling #4126 1:1000 MTCO1 (C4) - AbCam ab110413 1:1000 NDUFB8 (C1) - AbCam ab110413 1:1000 eNOS (NOS3) - Santa Cruz sc-654 1:1000 OCT1 - Santa Cruz sc-133866 1:500 PGC1α - AbCam ab54481 1:1000 UCP1 - Sigma U6382 1:2500 UQCRC2 (C3) - AbCam ab110413 1:1000 SDHB (C2) - AbCam ab110413 1:1000 Tubulin - Cell Signaling #2148 1:2000 ©2013 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0194/-/DC1 SUPPLEMENTARY DATA Supplementary Table 2. Primer sequences for qRT-PCR. Gene Accession number Forward primer Reverse primer Abca1 NM_013454.3 CCCAGAGCAAAAAGCGACTC GGTCATCATCACTTTGGTCCTTG Abcg5 NM_031884 TGTCCTACAGCGTCAGCAACC GGCCACTCTCGATGTACAAGG Abcg8 NM_026180 TCCTGTGAGCTGGGCATCCGA CCCGCAGCCTGAGCTCCCTAT Acaca NM_133360.2 CAGCTGGTGCAGAGGTACCG TCTACTCGCAGGTACTGCCG Acacb NM_133904.2 GCGCCTACTATGAGGCCCAGCA ACAAACTCGGCTGGGGACGC Acly NM_134037.2 -

Overexpression of ABCG5 and ABCG8 Promotes Biliary Cholesterol Secretion and Reduces Fractional Absorption of Dietary Cholestero
Overexpression of ABCG5 See the related Commentary beginning on page 605. and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol Liqing Yu,1 Jia Li-Hawkins,1 Robert E. Hammer,2,3 Knut E. Berge,1 Jay D. Horton,1 Jonathan C. Cohen,4 and Helen H. Hobbs1 1Department of Molecular Genetics and McDermott Center for Human Growth and Development, 2Department of Biochemistry, 3Howard Hughes Medical Institute, and 4Center for Human Nutrition, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas, USA Two ATP-binding cassette (ABC) transporters, ABCG5 and ABCG8, have been proposed to limit sterol absorption and to promote biliary sterol excretion in humans. To test this hypothesis, a P1 clone containing the human ABCG5 and ABCG8 genes was used to generate transgenic mice. The transgenes were expressed primarily in the liver and small intestine, mirroring the expression pattern of the endogenous genes. Transgene expression only modestly affected plasma and liver cholesterol levels but profoundly altered cholesterol transport. The fractional absorption of dietary cholesterol was reduced by about 50%, and biliary cholesterol levels were increased more than fivefold. Fecal neu- tral sterol excretion was increased three- to sixfold and hepatic cholesterol synthesis increased two- to fourfold in the transgenic mice. No significant changes in the pool size, composition, and fecal excretion of bile acids were observed in the transgenic mice. Transgene expression attenuated the increase in hepatic cholesterol content induced by consumption of a high cholesterol diet. These results demonstrate that increased expression of ABCG5 and ABCG8 selectively drives biliary neutral sterol secretion and reduces intestinal cholesterol absorption, leading to a selective increase in neu- tral sterol excretion and a compensatory increase in cholesterol synthesis. -

Apolipoprotein B
Laboratory Procedure Manual Analyte: Apolipoprotein B Matrix: Serum Method: Turbidimetric Assay on Roche Cobas® 6000 Method No.: Revised: As performed by: University of Minnesota – Advanced Research Diagnostics Laboratory (ARDL) Contact: Dr. Anthony Killeen, MD, PhD University of Minnesota Medical Center Fairview-University Medical Center University Campus Minneapolis, Minnesota Important Information for Users University of Minnesota – Advanced Research Diagnostics Laboratory (ARDL) periodically refines these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. Apolipoprotein B NHANES 2015-2016 Public Release Data Set Information This document details the Lab Protocol for testing the items listed in the following table: Data File Name Variable Name SAS Label LBXAPB Apolipoprotein B (mg/dL) APOB_I LBDAPBSI Apolipoprotein B (g/L) 2 of 20 Apolipoprotein B NHANES 2015-2016 1. SUMMARY OF TEST PRINCIPLE AND CLINICAL RELEVANCE A. Clinical Relevance Apolipoproteins are the protein constituents of the lipoproteins. Apolipoprotein B (Apo B) is the major protein component of low-density lipoprotein (LDL). About one-third of the LDL particles provide cholesterol to peripheral cells. The other two-thirds are metabolized by the liver. LDL-uptake in all of these cells occurs via LDL receptors. Apo B levels increase in hypercholesterolemia, pregnancy, LDL receptor defects, bile obstruction and nephrotic syndrome. Apo B levels decrease in liver disease, sepsis and estrogen administration. The combined measurement of apolipoprotein A-1 (Apo A1, present in HDL) and Apo B and the calculation of the Apo B:Apo A1 ratio can reflect a lipid metabolism disorder and the risk of developing atherosclerosis or coronary heart disease.