Guidelines for Bat Monitoring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Volume 41, 2000
BAT RESEARCH NEWS Volume 41 : No. 1 Spring 2000 I I BAT RESEARCH NEWS Volume 41: Numbers 1–4 2000 Original Issues Compiled by Dr. G. Roy Horst, Publisher and Managing Editor of Bat Research News, 2000. Copyright 2011 Bat Research News. All rights reserved. This material is protected by copyright and may not be reproduced, transmitted, posted on a Web site or a listserve, or disseminated in any form or by any means without prior written permission from the Publisher, Dr. Margaret A. Griffiths. The material is for individual use only. Bat Research News is ISSN # 0005-6227. BAT RESEARCH NEWS Volume41 Spring 2000 Numberl Contents Resolution on Rabies Exposure Merlin Tuttle and Thomas Griffiths o o o o eo o o o • o o o o o o o o o o o o o o o o 0 o o o o o o o o o o o 0 o o o 1 E - Mail Directory - 2000 Compiled by Roy Horst •••• 0 ...................... 0 ••••••••••••••••••••••• 2 ,t:.'. Recent Literature Compiled by :Margaret Griffiths . : ....••... •"r''• ..., .... >.•••••• , ••••• • ••< ...... 19 ,.!,..j,..,' ""o: ,II ,' f 'lf.,·,,- .,'b'l: ,~··.,., lfl!t • 0'( Titles Presented at the 7th Bat Researc:b Confei'ebee~;Moscow :i'\prill4-16~ '1999,., ..,, ~ .• , ' ' • I"',.., .. ' ""!' ,. Compiled by Roy Horst .. : .......... ~ ... ~· ....... : :· ,"'·~ .• ~:• .... ; •. ,·~ •.•, .. , ........ 22 ·.t.'t, J .,•• ~~ Letters to the Editor 26 I ••• 0 ••••• 0 •••••••••••• 0 ••••••• 0. 0. 0 0 ••••••• 0 •• 0. 0 •••••••• 0 ••••••••• 30 News . " Future Meetings, Conferences and Symposium ..................... ~ ..,•'.: .. ,. ·..; .... 31 Front Cover The illustration of Rhinolophus ferrumequinum on the front cover of this issue is by Philippe Penicaud . from his very handsome series of drawings representing the bats of France. -

7 Meeting of the Parties
Inf.EUROBATS.MoP7.45 7th Meeting of the Parties Brussels, Belgium, 15 – 17 September 2014 Report on Autecological Studies for Priority Species Convenor: Stéphane Aulagnier In accordance with Resolution 4.12, the current work being carried out on autecological studies of the Priority List of species (Rhinolophus euryale, Myotis capaccinii and Miniopterus schreibersii) should be updated by the Advisory Committee and should be made public. References of papers and reports dealing with autecological studies Rhinolophus euryale Barataud M., Jemin J., Grugier Y. & Mazaud S., 2009. Étude sur les territoires de chasse du Rhinolophe euryale, Rhinolophus euryale, en Corrèze, site Natura 2000 des Abîmes de la Fage. Le Naturaliste. Vendéen, 9 : 43-55. The cave of la Fage (Noailles, Département of Corrèze) is a major site for the Mediterranean horseshoe bat, Rhinolophus euryale Blasius 1853. However, contrary to the tendency to increase noted over the last 20 years in various other birth sites in France, numbers at la Fage have shown no change. One of the suspected causes links this to the presence of the A20 motorway, less than a kilometre away, where corpses have been collected. This article presents the results of a radio-tracking study of the space occupied by the colony, during the summers of 2006 and 2007. Voigt C.C., Schuller B.M., Greif S. & Siemers B.M., 2010. Perch-hunting in insectivorous Rhinolophus bats is related to the high energy costs of manoeuvring in flight. Journal of Comparative Physiology Biochemical Systemic and Environmental Physiology, 180(7): 1079-1088 Foraging behaviour of bats is supposedly largely influenced by the high costs of flapping flight. -

EU Action Plan for the Conservation of All Bat Species in the European Union
Action Plan for the Conservation of All Bat Species in the European Union 2018 – 2024 October 2018 Action Plan for the Conservation of All Bat Species in the European Union 2018 - 2024 EDITORS: BAROVA Sylvia (European Commission) & STREIT Andreas (UNEP/EUROBATS) COMPILERS: MARCHAIS Guillaume & THAURONT Marc (Ecosphère, France/The N2K Group) CONTRIBUTORS (in alphabetical order): BOYAN Petrov * (Bat Research & Conservation Centre, Bulgaria) DEKKER Jasja (Animal ecologist, Netherlands) ECOSPHERE: JUNG Lise, LOUTFI Emilie, NUNINGER Lise & ROUÉ Sébastien GAZARYAN Suren (EUROBATS) HAMIDOVIĆ Daniela (State Institute for Nature Protection, Croatia) JUSTE Javier (Spanish association for the study and conservation of bats, Spain) KADLEČÍK Ján (Štátna ochrana prírody Slovenskej republiky, Slovakia) KYHERÖINEN Eeva-Maria (Finnish Museum of Natural History, Finland) HANMER Julia (Bat Conservation Trust, United Kingdom) LEIVITS Meelis (Environmental Agency of the Ministry of Environment, Estonia) MARNELl Ferdia (National Parks & Wildlife Service, Ireland) PETERMANN Ruth (Federal Agency for Nature Conservation, Germany) PETERSONS Gunărs (Latvia University of Agriculture, Latvia) PRESETNIK Primož (Centre for Cartography of Fauna and Flora, Slovenia) RAINHO Ana (Institute for the Nature and Forest Conservation, Portugal) REITER Guido (Foundation for the protection of our bats in Switzerland) RODRIGUES Luisa (Institute for the Nature and Forest Conservation, Portugal) RUSSO Danilo (University of Napoli Frederico II, Italy) SCHEMBRI -

Index of Handbook of the Mammals of the World. Vol. 9. Bats
Index of Handbook of the Mammals of the World. Vol. 9. Bats A agnella, Kerivoula 901 Anchieta’s Bat 814 aquilus, Glischropus 763 Aba Leaf-nosed Bat 247 aladdin, Pipistrellus pipistrellus 771 Anchieta’s Broad-faced Fruit Bat 94 aquilus, Platyrrhinus 567 Aba Roundleaf Bat 247 alascensis, Myotis lucifugus 927 Anchieta’s Pipistrelle 814 Arabian Barbastelle 861 abae, Hipposideros 247 alaschanicus, Hypsugo 810 anchietae, Plerotes 94 Arabian Horseshoe Bat 296 abae, Rhinolophus fumigatus 290 Alashanian Pipistrelle 810 ancricola, Myotis 957 Arabian Mouse-tailed Bat 164, 170, 176 abbotti, Myotis hasseltii 970 alba, Ectophylla 466, 480, 569 Andaman Horseshoe Bat 314 Arabian Pipistrelle 810 abditum, Megaderma spasma 191 albatus, Myopterus daubentonii 663 Andaman Intermediate Horseshoe Arabian Trident Bat 229 Abo Bat 725, 832 Alberico’s Broad-nosed Bat 565 Bat 321 Arabian Trident Leaf-nosed Bat 229 Abo Butterfly Bat 725, 832 albericoi, Platyrrhinus 565 andamanensis, Rhinolophus 321 arabica, Asellia 229 abramus, Pipistrellus 777 albescens, Myotis 940 Andean Fruit Bat 547 arabicus, Hypsugo 810 abrasus, Cynomops 604, 640 albicollis, Megaerops 64 Andersen’s Bare-backed Fruit Bat 109 arabicus, Rousettus aegyptiacus 87 Abruzzi’s Wrinkle-lipped Bat 645 albipinnis, Taphozous longimanus 353 Andersen’s Flying Fox 158 arabium, Rhinopoma cystops 176 Abyssinian Horseshoe Bat 290 albiventer, Nyctimene 36, 118 Andersen’s Fruit-eating Bat 578 Arafura Large-footed Bat 969 Acerodon albiventris, Noctilio 405, 411 Andersen’s Leaf-nosed Bat 254 Arata Yellow-shouldered Bat 543 Sulawesi 134 albofuscus, Scotoecus 762 Andersen’s Little Fruit-eating Bat 578 Arata-Thomas Yellow-shouldered Talaud 134 alboguttata, Glauconycteris 833 Andersen’s Naked-backed Fruit Bat 109 Bat 543 Acerodon 134 albus, Diclidurus 339, 367 Andersen’s Roundleaf Bat 254 aratathomasi, Sturnira 543 Acerodon mackloti (see A. -

Mammal Survey Western Rhodopes Trigrad Bulgaria
MAMMAL SURVEY WESTERN RHODOPES TRIGRAD BULGARIA 2013 RAPPORT 2014.21 Nijmegen, juni 2014 Uitgave van de Veldwerkgroep van de Zoogdiervereniging 2 MAMMAL SURVEY WESTERN RHODOPES, TRIGRAD BULGARIA 2013 Chief Editors Lily Vercruijsse Kees Mostert Associate Editor Svetlana Miteva Authors Jan Boshamer Carolien van de Graaf Frank van der Knaap Ineke Kroes Kees Mostert Bart Noort Carola van den Tempel Lily Vercruijsse Jan Wondergem Translations Edu Goossens Eric Thomassen Maps Bart Noort Illustrations Jan Boshamer Ed Goossens Ineke Kroes Kees Mostert Lars Soerink Lily Vercruijsse David de Wit Uitgave van de Veldwerkgroep van de Zoogdiervereniging Rapport 2014.21 Nijmegen, juni 2014 ISBN/EAN 978-90-79924-34-9 3 Alle publicaties van de Veldwerkgroep kunnen kosteloos worden gedownload van de website: http://www.zoogdiervereniging.nl/veldwerkgroep All publications of the Field Study Group can be downloaded free of charge from the website: http://www.zoogdiervereniging.nl/fieldstudygroup 4 SUMMARY by Kees Mostert In the summer of 2013, the annual Field Study Group mammal study camp abroad took place (for the second time) in Bulgaria. This time we visited the Western Rhodopes, a karst landscape including mountain peaks reaching up to 2000 meters, steep and narrow valleys and extensive cave systems. A large part of this area is wooded, predominated with spruce forests, but also pastures at high altitude where sheep and cows graze. One of the interesting bird species in the area is the Capercaillie. The Bulgarian Society for the Protection of Birds has launched a project to preserve this species for the region. Research is being done on the habitat of the species, including a survey of plant and animal species. -

Kin Structure and Roost Fidelity in Greater Noctule Bats
bioRxiv preprint doi: https://doi.org/10.1101/675215; this version posted February 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Kin structure and roost fidelity in greater noctule bats João D. Santos1,2,*, Christoph F.J. Meyer1,3, Carlos Ibáñez4, Ana G. Popa-Lisseanu4 & Javier Juste4,5 1 Centre for Ecology, Evolution and Environmental Changes (cE3c), Faculty of Sciences, University of Lisbon, 1749-016 Lisbon, Portugal 2 UMR AGAP, CIRAD, F-34398 Montpellier, France 3 School of Science, Engineering and Environment, University of Salford, Salford, M5 4WT, United Kingdom 4 Department of Evolutionary Ecology, Estación Biológica de Doñana (CSIC), Avenida Américo Vespucio 26, 41092 Seville, Spain 5 Centro de Investigación Biomédica en Red de Epidemiología y Salud Pública, CIBERESP, Spain *Correspondent: João Santos, present address: Cirad, AGAP, TA A-108 / 03, Avenue Agropolis, 34398 Montpellier Cedex 5, France; E-mail: [email protected], Tel: +33623720645 Running Header: Kin structure of the greater noctule bioRxiv preprint doi: https://doi.org/10.1101/675215; this version posted February 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Santos et al. Kin structure and roost fidelity in giant noctule bats 1 ABSTRACT 2 Roost fidelity is an important aspect of mammalian biology. -

Nyctalus Lasiopterus, Nyctalus Aviator, Depredación, Paseriformes, Dieta, Migración, Generalista, Plumas, Excrementos
Nyctalus lasiopterus, Nyctalus aviator, depredación, paseriformes, dieta, migración, generalista, plumas, excrementos, amplificación. David Pastor Beviá Universitat d’Alacant Universidad de Alicante Ecología tróca del nóctulo grande (Nyctalus lasiopterus), un murciélago depredador de aves David Pastor Beviá David Pastor Beviá Escola de Doctorat Tesis doctoral Escuela de Doctorado ED|UA Alicante, mayo 2017 edua.ua.es Tesis doctoral Alicante, mayo 2017 Departamento de Ecología Facultad de Ciencias Ecología trófica del nóctulo grande (Nyctalus lasiopterus), un murciélago depredador de aves David Pastor Beviá Tesis presentada para aspirar al grado de DOCTOR POR LA UNIVERSIDAD DE ALICANTE DOCTORADO EN ANÁLISIS Y GESTIÓN DE ECOSISTEMAS Dirigida por: Carlos Ibáñez Ulargui, Javier Juste Ballesta Tutor académico: Germán López Iborra Parte de esta tesis ha sido financiada por el Ministerio de Industria, Comercio y Competitividad a través de los proyectos CGL 2009‐12393 y CGL2012‐38610. A mi familia Índice SECCIÓN INICIAL ………………………………………………………………………………………………………. 7 Síntesis …………………………………………………………………………………………………………………….. 9 Introducción General ………………………………………………………………………………………………. 19 SEGUNDA SECCIÓN …………………………………………………………………………………………………. 31 Artículos publicados ………………………………………………………………………………………………... 33 Chapter 1: A molecular approach to the study of avian DNA in bat faeces ………………. 35 Chapter 2: Concealed by darkness: interactions between predatory bats and nocturnally migrating songbirds illuminated by DNA sequencing…………………………….. 37 TERCERA SECCIÓN -

Greater Noctule Bat (Nyctalus Lasiopterus) IUCN Conservation Status
Greater noctule bat (Nyctalus lasiopterus) IUCN conservation status: Vulnerable - Local conservation status: Endangered The Greater noctule bat is Europe’s largest and rarest bat. It is primarily an arboreal species with the largest known colonies in Iberia being the nearby Los Alcornocales Natural Park with smaller breeding colonies in an urban park in Seville and a zoo in Jerez de la Frontera. It is known for supplementing its insectivorous diet with small passerine birds which they prey on at great heights during migration and is therefore only usually seen while emerging from their roosts or drinking, though this is also difficult seeing as they normally emerge once night has fallen. Noctule bats of the genus Nyctalus were known to occur in Gibraltar since 1967 with identification down to species level having been confirmed in 2015. European free-tailed bat (Tadarida teniotis) IUCN conservation status: Least Concern - Local conservation status: Vulnerable The European free-tailed bat is Europe’s second largest bat. Though abundant in numbers, it is a notoriously difficult species to study due to their roosting and feeding habits. Naturally a crevice dweller, they do not congregate in large colonies and have countless natural roosting spots available to them in Gibraltar. They are also high-fliers that rarely come down to drink as they get most of their moisture from the large airborne insects they consume. Isabelline serotine bat (Eptesicus isabellinus) IUCN conservation status: Least Concern - Local conservation status: Endangered The Isabelline serotine bat was first recorded in Gibraltar in 2013. The species has only recently been recognised as a full valid species following molecular analyses using mitochondrial DNA and nuclear markers, separating it from the Serotine bat (Eptesicus serotinus). -

Activity 6: Assessment of Species Diversity, Activity, Bat Habitats and Potential Threats
Activity 6: Assessment of species diversity, activity, bat habitats and potential threats In the frame of project: “Sustainable bats conservation in the cross border area” 1846 BatsConserve Representing BatMap /Elena Georgieva/ BatMap Sofia, 30.07.2019 Project co-funded by the European Union and National Funds of the participating countries *The contents of this publication are sole responsibility of project partners and can in no way be taken to reflect the views of the European Union, the participating countries, the Managing Authority and the Joint Secretariat. 1 Contents I. Developed spatial model that combines (based on) the spread of bat species, species diversity, habitats, activity and location of threats (e.g. highways, poorly managed forest areas, etc.). ................................................................................................................................... 4 II. Assessment of species biodiversity, activity, bat habitats and potential threats .......... 5 1. Assessment of species diversity ..................................................................................... 5 2. Activity ............................................................................................................................ 14 3. Bat habitats .................................................................................................................... 17 4. Potential threats ............................................................................................................ 22 III. Map material, coordinates -

Convention on the Conservation of Migratory Species of Wild Animals (CMS)
Convention on the Conservation of Migratory Species of Wild Animals (CMS) Secretariat provided by the United Nations Environment Programme (UNEP) Twelfth Meeting of the CMS Scientific Council Glasgow, Scotland, United Kingdom, 31 March-3 April 2004 CMS/ScC12/Inf.07 LIST OF COMMON NAMES OF SPECIES INCLUDED IN APPENDICES I AND II 1. The present document has been compiled by the Secretariat for its own purposes and, to the extent that it may also be useful to Scientific Council members and others, it is being reproduced here and circulated more widely. The list is updated after each Meeting of the Conference of the Parties to incorporate the latest amendments to the Appendices, making use of inter alia the amendment proposals submitted by Parties. These often provide the common names of the proposed species in several of the four languages included in the attached list (English, French, Spanish and German). 2. The Secretariat would be grateful for any suggestions to complete the missing entries (insofar as common names exist for the species in question) and/or any necessary corrections to the existing entries. These may be sent to the Secretariat ([email protected]) or left with the Secretariat during the meeting. For reasons of economy, documents are printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. Date of App Taxon English Français Español Deutsch Entry on Appendices I/II Accipitridae * eagles and hawks aigles, -

Bats, That Is Yet to Be Seen
Janice Pease (315)328-5793 [email protected] 130 Beebe Rd, Potsdam, N.Y. 13676 August 23, 2018 Via Email Honorable Kathleen H. Burgess, Secretary to the PSC Re: Case 16- F-0268, Application of Atlantic Wind LLC for a certificate of Environmental Compatibility and Public Need Pursuant to Article 10 for Construction of the North Ridge Wind Energy Project in the Towns of Parishville and Hopkinton, St. Lawrence County. Dear Secretary Burgess: Industrial wind is devastating to the bat populations, adding to the many factors which play a role in reducing their numbers worldwide. While the wind industry likes to suggest that the advantage turbines provide to help reduce climate change (inadvertently benefitting all creatures) far outweighs their negative impact on the bats, that is yet to be seen. The data is simply not available to calculate the environmental/financial net losses accurately. With industrial wind’s intermittent and unreliable energy, the advantages are not nearly as the wind lobbyists suggest. The fragmentation/depletion of critical habitat due to wind turbines massive land use affects all animal species reliant on that space, ricocheting down the food chain. The loss of habitat as well as loss of carbon-sinks make the industrial turbine a very unlikely savior for any species. The net loss has simply not been calculated. Farmers are easily drawn into the debate by hosting these “farms”, while receiving large financial payouts. These same farmers are seemingly unaware of the immense benefit that bats provide by eating insect pests, saving farmers billions/year. The weakening of the local ecosystems will most certainly result in lower crop yields as well as contribute to financial losses as well. -
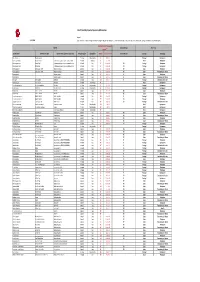
LEPI 2020 Pivot Table VF 20210406 (Version 1).Xlsb
List of Potentially Impacted Species by EDP activities Legend: 31/12/2020 (1) threat levels: i. Critically endangered (CR); ii. Endangered (EN); iii. Vulnerable (VU); iv. Near threatened (NT), Least concern (LC), Data deficient (DD), Not Applicable (NA) and Not Evaluated (NE) IUCN Red List of Threatened Species National Red List Occurrence Species (1) Scientific name Common name (EN) Common name (country of occurrence) Reino/Kingdom Classe/Class 2020‐01 latest assessment National Red List Country Tecnology Dianthus marizii Dianthus marizii ‐ Plantae Magnoliopsida LC 6‐abr‐11 Portugal Hydropower Coendou prehensilis Brazilian Porcupine ouriço‐cacheiro, porco‐espinho, cuandu e cuim Animalia Mammalia LC 10‐jun‐16 Brazil Windpower Hieraetus pennatus Booted Eagle A águia‐pequena, águia‐calçada ou águia‐de‐botas Animalia Aves LC 31‐mar‐15 NT Portugal Windpower Hieraetus pennatus Booted Eagle A águia‐pequena, águia‐calçada ou águia‐de‐botas Animalia Aves LC 31‐mar‐15 NT Portugal Hydropower Merops apiaster European Bee‐eater Abejaruco europeo Animalia Aves LC 31‐mar‐15 NE Spain Windpower Merops apiaster European Bee‐eater Abejaruco europeo Animalia Aves LC 31‐mar‐15 NE Spain Transmision grid‐ Renew Pernis apivorus Abejero europeo Animalia Aves LC 1‐out‐16 LC Spain Windpower Pernis apivorus Abejero europeo Animalia Aves LC 1‐out‐16 LC Spain Transmision grid‐ Renew Otis tarda Great bustard Abetarda Animalia Aves VU 1‐out‐17 EN Portugal Distribution Grid ‐activ Oxydoras niger ripsaw catfish Abotoado Animalia Actinopterygii NE Brazil Hydropower Santolina