George Pake Became Purcell’S Second Student
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development of Military Nuclear Strategy And
The Development of Military Nuclear Strategy and Anglo-American Relations, 1939 – 1958 Submitted by: Geoffrey Charles Mallett Skinner to the University of Exeter as a thesis for the degree of Doctor of Philosophy in History, July 2018 This thesis is available for Library use on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. I certify that all material in this thesis which is not my own work has been identified and that no material has previously been submitted and approved for the award of a degree by this or any other University. (Signature) ……………………………………………………………………………… 1 Abstract There was no special governmental partnership between Britain and America during the Second World War in atomic affairs. A recalibration is required that updates and amends the existing historiography in this respect. The wartime atomic relations of those countries were cooperative at the level of science and resources, but rarely that of the state. As soon as it became apparent that fission weaponry would be the main basis of future military power, America decided to gain exclusive control over the weapon. Britain could not replicate American resources and no assistance was offered to it by its conventional ally. America then created its own, closed, nuclear system and well before the 1946 Atomic Energy Act, the event which is typically seen by historians as the explanation of the fracturing of wartime atomic relations. Immediately after 1945 there was insufficient systemic force to create change in the consistent American policy of atomic monopoly. As fusion bombs introduced a new magnitude of risk, and as the nuclear world expanded and deepened, the systemic pressures grew. -

Copyright by Paul Harold Rubinson 2008
Copyright by Paul Harold Rubinson 2008 The Dissertation Committee for Paul Harold Rubinson certifies that this is the approved version of the following dissertation: Containing Science: The U.S. National Security State and Scientists’ Challenge to Nuclear Weapons during the Cold War Committee: —————————————————— Mark A. Lawrence, Supervisor —————————————————— Francis J. Gavin —————————————————— Bruce J. Hunt —————————————————— David M. Oshinsky —————————————————— Michael B. Stoff Containing Science: The U.S. National Security State and Scientists’ Challenge to Nuclear Weapons during the Cold War by Paul Harold Rubinson, B.A.; M.A. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin August 2008 Acknowledgements Thanks first and foremost to Mark Lawrence for his guidance, support, and enthusiasm throughout this project. It would be impossible to overstate how essential his insight and mentoring have been to this dissertation and my career in general. Just as important has been his camaraderie, which made the researching and writing of this dissertation infinitely more rewarding. Thanks as well to Bruce Hunt for his support. Especially helpful was his incisive feedback, which both encouraged me to think through my ideas more thoroughly, and reined me in when my writing overshot my argument. I offer my sincerest gratitude to the Smith Richardson Foundation and Yale University International Security Studies for the Predoctoral Fellowship that allowed me to do the bulk of the writing of this dissertation. Thanks also to the Brady-Johnson Program in Grand Strategy at Yale University, and John Gaddis and the incomparable Ann Carter-Drier at ISS. -

Annual Report 2013.Pdf
ATOMIC HERITAGE FOUNDATION Preserving & Interpreting Manhattan Project History & Legacy preserving history ANNUAL REPORT 2013 WHY WE SHOULD PRESERVE THE MANHATTAN PROJECT “The factories and bombs that Manhattan Project scientists, engineers, and workers built were physical objects that depended for their operation on physics, chemistry, metallurgy, and other nat- ural sciences, but their social reality - their meaning, if you will - was human, social, political....We preserve what we value of the physical past because it specifically embodies our social past....When we lose parts of our physical past, we lose parts of our common social past as well.” “The new knowledge of nuclear energy has undoubtedly limited national sovereignty and scaled down the destructiveness of war. If that’s not a good enough reason to work for and contribute to the Manhattan Project’s historic preservation, what would be? It’s certainly good enough for me.” ~Richard Rhodes, “Why We Should Preserve the Manhattan Project,” Bulletin of the Atomic Scientists, May/June 2006 Photographs clockwise from top: J. Robert Oppenheimer, General Leslie R. Groves pinning an award on Enrico Fermi, Leona Woods Marshall, the Alpha Racetrack at the Y-12 Plant, and the Bethe House on Bathtub Row. Front cover: A Bruggeman Ranch property. Back cover: Bronze statues by Susanne Vertel of J. Robert Oppenheimer and General Leslie Groves at Los Alamos. Table of Contents BOARD MEMBERS & ADVISORY COMMITTEE........3 Cindy Kelly, Dorothy and Clay Per- Letter from the President..........................................4 -

National Academy of Sciences July 1, 1979 Officers
NATIONAL ACADEMY OF SCIENCES JULY 1, 1979 OFFICERS Term expires President-PHILIP HANDLER June 30, 1981 Vice-President-SAUNDERS MAC LANE June 30, 1981 Home Secretary-BRYCE CRAWFORD,JR. June 30, 1983 Foreign Secretary-THOMAS F. MALONE June 30, 1982 Treasurer-E. R. PIORE June 30, 1980 Executive Officer Comptroller Robert M. White David Williams COUNCIL Abelson, Philip H. (1981) Markert,C. L. (1980) Berg, Paul (1982) Nierenberg,William A. (1982) Berliner, Robert W. (1981) Piore, E. R. (1980) Bing, R. H. (1980) Ranney, H. M. (1980) Crawford,Bryce, Jr. (1983) Simon, Herbert A. (1981) Friedman, Herbert (1982) Solow, R. M. (1980) Handler, Philip (1981) Thomas, Lewis (1982) Mac Lane, Saunders (1981) Townes, Charles H. (1981) Malone, Thomas F. (1982) Downloaded by guest on September 30, 2021 SECTIONS The Academyis divided into the followingSections, to which membersare assigned at their own choice: (11) Mathematics (31) Engineering (12) Astronomy (32) Applied Biology (13) Physics (33) Applied Physical and (14) Chemistry Mathematical Sciences (15) Geology (41) Medical Genetics Hema- (16) Geophysics tology, and Oncology (21) Biochemistry (42) Medical Physiology, En- (22) Cellularand Develop- docrinology,and Me- mental Biology tabolism (23) Physiological and Phar- (43) Medical Microbiology macologicalSciences and Immunology (24) Neurobiology (51) Anthropology (25) Botany (52) Psychology (26) Genetics (53) Social and Political Sci- (27) Population Biology, Evo- ences lution, and Ecology (54) Economic Sciences In the alphabetical list of members,the numbersin parentheses, followingyear of election, indicate the respective Class and Section of the member. CLASSES The members of Sections are grouped in the following Classes: I. Physical and Mathematical Sciences (Sections 11, 12, 13, 14, 15, 16). -

Conformational Transition in Immunoglobulin MOPC 460" by Correction. in Themembership List of the National Academy of Scien
Corrections Proc. Natl. Acad. Sci. USA 74 (1977) 1301 Correction. In the article "Kinetic evidence for hapten-induced Correction. In the membership list of the National Academy conformational transition in immunoglobulin MOPC 460" by of Sciences that appeared in the October 1976 issue of Proc. D. Lancet and I. Pecht, which appeared in the October 1976 Natl. Acad. Sci. USA 73,3750-3781, please note the following issue of Proc. Nati. Acad. Sci. USA 73,3549-3553, the authors corrections: H. E. Carter, Britton Chance, Seymour S. Cohen, have requested the following changes. On p. 3550, right-hand E. A. Doisy, Gerald M. Edelman, and John T. Edsall are affil- column, second line from bottom, and p. 3551, left-hand col- iated with the Section ofBiochemistry (21), not the Section of umn, fourth line from the top, "Fig. 2" should be "Fig. 1A." Botany (25). In the legend of Table 2, third line, note (f) should read "AG, = -RTlnKj." On p. 3553, left-hand column, third paragraph, fifth line, "ko" should be replaced by "Ko." Correction. In the Author Index to Volume 73, January-De- cember 1976, which appeared in the December 1976 issue of Proc. Natl. Acad. Sci. USA 73, 4781-4788, the limitations of Correction. In the article "Amino-terminal sequences of two computer alphabetization resulted in the listing of one person polypeptides from human serum with nonsuppressible insu- as the author of another's paper. On p. 4786, it should indicate lin-like and cell-growth-promoting activities: Evidence for that James Christopher Phillips had an article beginning on p. -

The People Who Invented the Internet Source: Wikipedia's History of the Internet
The People Who Invented the Internet Source: Wikipedia's History of the Internet PDF generated using the open source mwlib toolkit. See http://code.pediapress.com/ for more information. PDF generated at: Sat, 22 Sep 2012 02:49:54 UTC Contents Articles History of the Internet 1 Barry Appelman 26 Paul Baran 28 Vint Cerf 33 Danny Cohen (engineer) 41 David D. Clark 44 Steve Crocker 45 Donald Davies 47 Douglas Engelbart 49 Charles M. Herzfeld 56 Internet Engineering Task Force 58 Bob Kahn 61 Peter T. Kirstein 65 Leonard Kleinrock 66 John Klensin 70 J. C. R. Licklider 71 Jon Postel 77 Louis Pouzin 80 Lawrence Roberts (scientist) 81 John Romkey 84 Ivan Sutherland 85 Robert Taylor (computer scientist) 89 Ray Tomlinson 92 Oleg Vishnepolsky 94 Phil Zimmermann 96 References Article Sources and Contributors 99 Image Sources, Licenses and Contributors 102 Article Licenses License 103 History of the Internet 1 History of the Internet The history of the Internet began with the development of electronic computers in the 1950s. This began with point-to-point communication between mainframe computers and terminals, expanded to point-to-point connections between computers and then early research into packet switching. Packet switched networks such as ARPANET, Mark I at NPL in the UK, CYCLADES, Merit Network, Tymnet, and Telenet, were developed in the late 1960s and early 1970s using a variety of protocols. The ARPANET in particular led to the development of protocols for internetworking, where multiple separate networks could be joined together into a network of networks. In 1982 the Internet Protocol Suite (TCP/IP) was standardized and the concept of a world-wide network of fully interconnected TCP/IP networks called the Internet was introduced. -
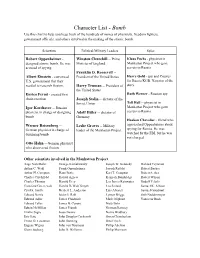
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I. -

Edward B. Van Vleck 1863‐1943
Remembering Van: Three Madison families and other tales “Van had many enthusiasms and loyalties, but none exceeded those he felt for the University of Wisconsin and its home, Madison.” 1 Charles Van Hise Born in 1857, Charles Van Hise was a distinguished geologist who had been elected to the National Academy of Sciences in 1902. In 1903, he was named President of the University of Wisconsin 2 In 1904, Van Hise recruited Charles Russell Bardeen, a member of the first class to graduate of the Johns Hopkins Medical School, to found a medical school at the University of Wisconsin John Bardeen is at the right hand end. His grandfather, Charles W Bardeen, is second from the left. 3 Charles Sumner Slichter 1864-1946 In 1906, Van Hise appointed Charles S. Slichter head of the math department. An applied mathematician with interests in flow of liquids through porous media, Slichter realized the department needed to strengthen the faculty in pure mathematics. 4 Edward B. Van Vleck 1863‐1943 Edward B Van Vleck, had earned a PhD in mathematics at Gottingen in1893, and was a member of the National Academy of Sciences. Slichter’s first action as department head was to recruit Van Vleck to bring strength in pure mathematics. 5 John Hasbrouck Van Vleck was born in 1899. His interest in trains began at a early age. Van attended his first Wisconsin football game at age 10.(Wisconsin vs Minnesota) It was the first game at which the band played the famous song “On Wisconsin”. As an undergraduate at Wisconsin, Van played flute In the band. -

0X0a I Don't Know Gregor Weichbrodt FROHMANN
0x0a I Don’t Know Gregor Weichbrodt FROHMANN I Don’t Know Gregor Weichbrodt 0x0a Contents I Don’t Know .................................................................4 About This Book .......................................................353 Imprint ........................................................................354 I Don’t Know I’m not well-versed in Literature. Sensibility – what is that? What in God’s name is An Afterword? I haven’t the faintest idea. And concerning Book design, I am fully ignorant. What is ‘A Slipcase’ supposed to mean again, and what the heck is Boriswood? The Canons of page construction – I don’t know what that is. I haven’t got a clue. How am I supposed to make sense of Traditional Chinese bookbinding, and what the hell is an Initial? Containers are a mystery to me. And what about A Post box, and what on earth is The Hollow Nickel Case? An Ammunition box – dunno. Couldn’t tell you. I’m not well-versed in Postal systems. And I don’t know what Bulk mail is or what is supposed to be special about A Catcher pouch. I don’t know what people mean by ‘Bags’. What’s the deal with The Arhuaca mochila, and what is the mystery about A Bin bag? Am I supposed to be familiar with A Carpet bag? How should I know? Cradleboard? Come again? Never heard of it. I have no idea. A Changing bag – never heard of it. I’ve never heard of Carriages. A Dogcart – what does that mean? A Ralli car? Doesn’t ring a bell. I have absolutely no idea. And what the hell is Tandem, and what is the deal with the Mail coach? 4 I don’t know the first thing about Postal system of the United Kingdom. -

William T. Golden October 1950 – April 1951
Impacts of the Early Cold War on the Formulation of U.S. Science Policy Selected Memoranda of William T. Golden October 1950 – April 1951 Edited with an Appreciation by William A. Blanpied Foreward by Neal Lane Copyright © 1995, 2000 American Association for the Advancement of Science 1200 New York Avenue, NW, Washington, DC 20005 The findings, conclusions, and opinions stated or implied in this publication are those of the authors. They do not necessarily reflect the views of the Board of Directors, Council, or membership of the American Association for the Advancement of Science. William T. Golden Contents Contents Foreword....................................................................................................................................................... 4 Preface ......................................................................................................................................................... 6 Acknowledgments ........................................................................................................................................ 7 A Brief Biography ........................................................................................................................................ 8 William T. Golden’s Chronicle of an Era: An Appreciation ....................................................................... 9 Decision Memorandum F.J. Lawton, Decision Memorandum for the President, October 19, 1950................................................ 34 Conversations: 1950 Herman -

Highlights International Physics Community Joins Forces for 2005 World Conference in South Africa
June 2004 NEWS Volume 13, No. 6 A Publication of The American Physical Society http://www.aps.org/apsnews International Physics Community Joins Forces for Innovation Task Force Unveils New 2005 World Conference in South Africa Advocacy Campaign On April 20, at a press confer- As part of the celebration of the made to society in the past, and Several international confer- America, and the Middle East, as well ence in Washington, DC, leaders World Year of Physics 2005, formulate a plan for the contribu- ences have been scheduled for as from more developed countries, from industry and academia UNESCO, ICTP, IUPAP and the tions that it can and should make 2004 on these topics, and will serve and the organizers hope to be able unveiled an advocacy campaign South African Institute of Physics in the future. The conference is as preparatory meetings for the to provide travel grants for as many to illustrate the importance of (SAIP) will sponsor a World Confer- partially a follow-up to a broader 2005 World Conference—the first as half of the attendees. basic research to the future of ence on Physics and Sustainable United Nations World Summit on time the international physics com- “Physics has contributed greatly American innovation, economic Development, to be held October Sustainable Development held in munity will focus its collective to the health and economic well growth, and job creation. 31-November 2, 2005, in Durban, Johannesburg in the summer of attention on these themes, and the being of people around the world. Targeted at policy makers and South Africa. -

By Senator E
SENATE FLOOR speech b y senator d an iel k . in ouy e 1963/1964 Release Time: Mr. President, The greatest wrong our nation has ever perpetrated, in a history perhaps unmatched for honor and generosity towards mankind, is one we have inflicted on a part of our own people. Since the days when the settlers first came to this new and wild land, Americans have been consumed with an idea that departed entirely from the thought and custom of all previous history. This idea involved a new way of looking at the character of man. Our government, also for the first time in history, was built on this principle. As Abraham Lincoln once said: "Most governments have been based, practically, on the denial of the equal rights of men. Ours began by affirming those rights* They said ’some men are too ignorant and vicious to share in government. 'Possibly so, ’ said we, ’and by your system you would always keep them ignorant and vicious. We propose to give all a chance; and we expect the weak to grow stronger, the ignorant wiser, and all better and happier together'." Yet here in the first country of the world which was dedicated to this idea, in the country which has been responsible for keeping it alive in times of darkness ever since, and is responsible today, we have not worked or cared to see it scrupulously applied among our own people. We have given reasons, some of us, and others have closed their eyes and turned their backs upon laws, practices and attitudes which we should long ago have fought to have abolished.