Structure, Properties and Preparation of Perovskite-Type Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Safety Data Sheet CS: 1.7.2
Safety Data Sheet CS: 1.7.2 Page : 1 of 6 Infosafe No ™ 1CH9H Issue Date : July 2018 RE-ISSUED by CHEMSUPP Product Name : LEAD (II,IV) OXIDE Classified as hazardous 1. Identification GHS Product LEAD (II,IV) OXIDE Identifier Company Name CHEM-SUPPLY PTY LTD (ABN 19 008 264 211) Address 38 - 50 Bedford Street GILLMAN SA 5013 Australia Telephone/Fax Tel: (08) 8440-2000 Number Fax: (08) 8440-2001 Recommended use Storage batteries, glass, pottery and enameling, varnish, purification of alcohol, packing pipe joints, of the chemical and metal protective paints, fluxes, ceramic glazes and laboratory reagent. restrictions on use Other Names Name Product Code Lead oxide red Red lead LEAD (II,IV) OXIDE LR LL027 Lead tetraoxide LEAD (II,IV) OXIDE TG LT027 Other Information EMERGENCY CONTACT NUMBER: +61 08 8440 2000 Business hours: 8:30am to 5:00pm, Monday to Friday. Chem-Supply Pty Ltd does not warrant that this product is suitable for any use or purpose. The user must ascertain the suitability of the product before use or application intended purpose. Preliminary testing of the product before use or application is recommended. Any reliance or purported reliance upon Chem-Supply Pty Ltd with respect to any skill or judgement or advice in relation to the suitability of this product of any purpose is disclaimed. Except to the extent prohibited at law, any condition implied by any statute as to the merchantable quality of this product or fitness for any purpose is hereby excluded. This product is not sold by description. Where the provisions of Part V, Division 2 of the Trade Practices Act apply, the liability of Chem-Supply Pty Ltd is limited to the replacement of supply of equivalent goods or payment of the cost of replacing the goods or acquiring equivalent goods. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
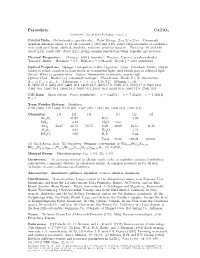
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy. -

Polymorphism in Bi-Based Perovskite Oxides: a First-Principles Study
Polymorphism in Bi-based Perovskite Oxides: a First-principles Study Oswaldo Diéguez Department of Materials Science and Engineering, Tel Aviv University, Email: [email protected] Web: http://www.eng.tau.ac.il/~dieguez Abstract Under normal conditions, bulk crystals of BiScO3, BiCrO3, BiMnO3, BiFeO3, and BiCoO3 present three very different variations of the perovskite structure: an antipolar phase, a rhombohedral phase with a large polarization along the space diagonal of the pseudocubic unit cell, and a supertetragonal phase with even larger polarization. With the aim of understanding the causes for this variety, we have used a genetic algorithm to search for minima in the surface energy of these materials. Our results show that the number of these minima is very large when compared to that of typical ferroelectric perovskites like BaTiO3 and PbTiO3, and that a fine energy balance between them results in the large structural differences seen. As byproducts of our search we have identified charge-ordering structures with low energy in BiMnO3, and several phases with energies that are similar to that of the ground state of BiCrO3. We have also found that a inverse supertetragonal phase exists in bulk, likely to be favored in films epitaxially grown at large values of tensile misfit strain. Short Biography Dr. Oswaldo Dieguez received his PhD from University of Santiago de Compostela, Spain. After postdoctoral stays at Cambridge, Rutgers, and MIT he joined the Institute of Materials Science of Barcelona as a staff researcher. He moved to the Department of Materials Science and Engineering of Tel Aviv University in 2013, where he is a senior lecturer (assistant professor). -

Aurivillius Halide Perovskite: a New Family of Two-Dimensional Materials for Optoelectronic Applications
Aurivillius halide perovskite: a new family of two-dimensional materials for optoelectronic applications Shuai Zhaoa,*, Chunfeng Lanb, Huanhuan Lia, Chu Zhangc, Tingli Mac,d aChongqing Key Laboratory of Green Energy Materials Technology and Systems, School of Science, Chongqing University of Technology, Chongqing 400054, P.R. China bSchool of Automotive and Transportation Engineering, Shenzhen Polytechnic, Shenzhen 518055, P.R. China cDepartment of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, P. R. China dGraduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, Kitakyushu, Fukuoka 808-0196, Japan *E-mail: [email protected] Abstract Layered perovskites have attracted considerable attention in optoelectronic applications due to their excellent electronic properties and stability. In this work, the quasi-2D aurivillius halide perovskites are investigated using density functional theory. The single-layer aurivillius perovskite Ba2PbI6 is predicted to have a direct bandgap of 1.89 eV, which is close to that of the Ruddlesden–Popper perovskite Cs2PbI4. The electronic structures near the Fermi level are mainly governed by the [PbX6] octahedra, which leads to similar electronic properties to that of Cs2PbI4. Decomposition energies reveal that these aurivillius perovskites exhibit thermal instability. Increasing the number of [PbX6] octahedra layer can enhance the stability and reduce the bandgap. Bi- and In-based aurivillius perovskites are also calculated to evaluate the Pb-free alternatives. These calculations can serve as a theoretical support in exploring novel layered perovskites. Introduction Since the first report of organic–inorganic halide perovskite (OIHP) solar cells in 2009, the power conversion efficiency (PCE) has dramatically increased to 25.02%.1–3 OIHP exhibits unprecedented optoelectronic properties, such as high carrier mobility, large optical absorption coefficient, and low-cost fabrication method.4–6 Thus, they are promising absorber materials of next-generation solar cells. -

Metal Oxide Compact Electron Transport Layer Modification For
materials Review Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells Md. Shahiduzzaman 1,* , Shoko Fukaya 2, Ersan Y. Muslih 3, Liangle Wang 2 , Masahiro Nakano 3, Md. Akhtaruzzaman 4, Makoto Karakawa 1,2,3, Kohshin Takahashi 3, Jean-Michel Nunzi 1,5 and Tetsuya Taima 1,2,3,* 1 Nanomaterials Research Institute, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan; karakawa@staff.kanazawa-u.ac.jp (M.K.); [email protected] (J.-M.N.) 2 Graduate School of Frontier Science Initiative, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan; [email protected] (S.F.); [email protected] (L.W.) 3 Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan; [email protected] (E.Y.M.); [email protected] (M.N.); [email protected] (K.T.) 4 Solar Energy Research Institute, The National University of Malaysia, Bangi 43600, Malaysia; [email protected] 5 Department of Physics, Engineering Physics and Astronomy, Queens University, Kingston, ON K7L-3N6, Canada * Correspondence: [email protected] (M.S.); [email protected] (T.T.); Tel.: +81-76-234-4937 (M.S.) Received: 14 April 2020; Accepted: 9 May 2020; Published: 11 May 2020 Abstract: Perovskite solar cells (PSCs) have appeared as a promising design for next-generation thin-film photovoltaics because of their cost-efficient fabrication processes and excellent optoelectronic properties. However, PSCs containing a metal oxide compact layer (CL) suffer from poor long-term stability and performance. The quality of the underlying substrate strongly influences the growth of the perovskite layer. -

Monoanionic Tin Oligomers Featuring Sn–Sn Or Sn–Pb Bonds: Synthesis and Characterization of a Tris(Triheteroarylstannyl)Stannate and -Plumbate
inorganics Communication Monoanionic Tin Oligomers Featuring Sn–Sn or Sn–Pb Bonds: Synthesis and Characterization of a Tris(Triheteroarylstannyl)Stannate and -Plumbate Kornelia Zeckert Institute of Inorganic Chemistry, University of Leipzig, Johannisallee 29, D-04103 Leipzig, Germany; [email protected]; Tel.: +49-341-9736-130 Academic Editor: Axel Klein Received: 20 May 2016; Accepted: 14 June 2016; Published: 20 June 2016 6OtBu 6OtBu Abstract: The reaction of the lithium tris(2-pyridyl)stannate [LiSn(2-py )3] (py = C5H3N-6-OtBu), 6OtBu 1, with the element(II) amides E{N(SiMe3)2}2 (E = Sn, Pb) afforded complexes [LiE{Sn(2-py )3}3] for E = Sn (2) and E = Pb (3), which reveal three Sn–E bonds each. Compounds 2 and 3 have been characterized by solution NMR spectroscopy and X-ray crystallographic studies. Large 1J(119Sn–119/117Sn) as well as 1J(207Pb–119/117Sn) coupling constants confirm their structural integrity in solution. However, contrary to 2, complex 3 slowly disintegrates in solution to give elemental lead 6OtBu and the hexaheteroarylditin [Sn(2-py )3]2 (4). Keywords: tin; lead; catenation; pyridyl ligands 1. Introduction The synthesis and characterization of catenated heavier group 14 element compounds have attracted attention in recent years [1–5]. However, contrary to silicon and germanium, there are limitations for tin and lead associated with the significant decrease in element–element bond energy. Hence, homonuclear as well as heteronuclear molecules with E–E bonds become less stable when E represents tin and or lead. Moreover, within this class of compounds, discrete branched oligomers with more than one E–E bond are rare compared with their linear analogs [6–10]. -

Tetraethyllead Is a Deadly Toxic Chemical Substance Giving Rise to Severe Psychotic Manifestations. for Its Excellent Properties
Industrial Health, 1986, 24, 139-150. Determination of Triethyllead, Diethyllead and Inorganic Lead in Urine by Atomic Absorption Spectrometry Fumio ARAI Department of Public Health St. Marianna University School of Medicine 2095 Sugao, Miyamae-ku, Kawasaki 213, Japan (Received March 10, 1986 and in revised form May 21, 1986) Abstract : A method was developed for the sequential extraction of tetraethyllead (Et4Pb), triethyllead (Et3Pb+), diethyllead (Et2Pb2+) and inorganic lead (Pb2+) from one urine sample with methyl isobutyl ketone and the subsequent sequential determination of the respective species of lead by flame and flameless atomic ab- sorption spectrometry. When 40 ml of a urine sample to which 2 ƒÊg of Pb of each of Et4Pb, Et3Pb+, Et2Pb2+ or Pb2+ had been experimentally added was assayed for the respective species of lead by flame atomic absorption spectrometry, ten repetitions of the assay gave a mean recovery rate of 98% for each of Et4Pb, Et3Pb+, and Et2Pb2+, and 99% for Pb2+, with a coefficient of variation of 2.0% for Et4Pb, 0.7% for Et3Pb+ and Pb2+, 2.6% for Et2Pb2+, and a detection limit of 4 ƒÊg of Pb/L for Et4Pb, 3 ƒÊg of Pb/L for Et3Pb+, and 5 ƒÊg of Pb/L for each of Et2Pb2+ and Pb2+. Examination of urine samples from a patient with tetraethyllead poisoning 22 days after exposure to the lead revealed that the total lead output was made up of about 51% Pb2+, about 43% Et2Pb2+, and about 6% Et3Pb+ but no Et4Pb. Ad- ministration of calcium ethylenediaminetetraacetic acid (Ca-EDTA) was followed by no increased urinary excretion of Et3Pb+ or Et2Pb2+.