Vibrational and Rotational Spectroscopy of Diatomic Molecules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vibrational-Rotational Spectroscopy ( )
Vibrational-Rotational Spectroscopy Vibrational-Rotational Spectrum of Heteronuclear Diatomic Absorption of mid-infrared light (~300-4000 cm-1): • Molecules can change vibrational and rotational states • Typically at room temperature, only ground vibrational state populated but several rotational levels may be populated. • Treating as harmonic oscillator and rigid rotor: subject to selection rules ∆v = ±1 and ∆J = ±1 EEEfield=∆ vib +∆ rot =ω =−EEfi = EvJEvJ()′,, ′ −( ′′ ′′ ) ω 11 vvvBJJvvBJJ==00()′′′′′′′′′ +++−++()11() () + 2πc 22 At room temperature, typically v=0′′ and ∆v = +1: ′ ′ ′′ ′′ vv=+0 BJJ() +−11 JJ( +) Now, since higher lying rotational levels can be populated, we can have: ∆=+J 1 JJ′′′=+1 vv= ++21 BJ( ′′ ) R − branch 0 P− branch ∆=−J 1 JJ′′′=−1 vv=−0 2 BJ′′ J’=4 J’=3 J’=1 v’=1 J’=0 P branch Q branch J’’=3 12B J’’=2 6B J’’=1 2B v’’=0 J’’=0 EJ” =0 2B 2B 4B 2B 2B 2B -6B -4B -2B +2B +4B +8B ν ν 0 By measuring absorption splittings, we can get B . From that, the bond length! In polyatomics, we can also have a Q branch, where ∆J0= and all transitions lie at ν=ν0 . This transition is allowed for perpendicular bands: ∂µ ∂q ⊥ to molecular symmetry axis. Intensity of Vibrational-Rotational Transitions There is generally no thermal population in upper (final) state (v’,J’) so intensity should scale as population of lower J state (J”). ∆=NNvJNvJ(,′ ′′′′′′′ ) − (, ) ≈ NJ ( ) NJ()′′∝− gJ ()exp( ′′ EJ′′ / kT ) =+()21exp(J′′ −hcBJ ′′() J ′′ + 1/) kT 5.33 Lecture Notes: Vibrational-Rotational Spectroscopy Page 2 Rotational Populations at Room Temperature for B = 5 cm-1 gJ'' thermal population NJ'' 0 5 10 15 20 Rotational Quantum Number J'' So, the vibrational-rotational spectrum should look like equally spaced lines about ν 0 with sidebands peaked at J’’>0. -

Rotational Motion (The Dynamics of a Rigid Body)
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Robert Katz Publications Research Papers in Physics and Astronomy 1-1958 Physics, Chapter 11: Rotational Motion (The Dynamics of a Rigid Body) Henry Semat City College of New York Robert Katz University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/physicskatz Part of the Physics Commons Semat, Henry and Katz, Robert, "Physics, Chapter 11: Rotational Motion (The Dynamics of a Rigid Body)" (1958). Robert Katz Publications. 141. https://digitalcommons.unl.edu/physicskatz/141 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Robert Katz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 11 Rotational Motion (The Dynamics of a Rigid Body) 11-1 Motion about a Fixed Axis The motion of the flywheel of an engine and of a pulley on its axle are examples of an important type of motion of a rigid body, that of the motion of rotation about a fixed axis. Consider the motion of a uniform disk rotat ing about a fixed axis passing through its center of gravity C perpendicular to the face of the disk, as shown in Figure 11-1. The motion of this disk may be de scribed in terms of the motions of each of its individual particles, but a better way to describe the motion is in terms of the angle through which the disk rotates. -
Optical Spectroscopy - Processes Monitored UV/ Fluorescence/ IR/ Raman/ Circular Dichroism
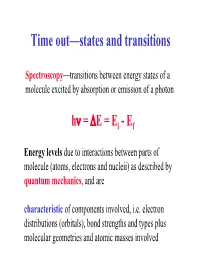
Time out—states and transitions Spectroscopy—transitions between energy states of a molecule excited by absorption or emission of a photon hν = ∆E = Ei -Ef Energy levels due to interactions between parts of molecule (atoms, electrons and nucleii) as described by quantum mechanics, and are characteristic of components involved, i.e. electron distributions (orbitals), bond strengths and types plus molecular geometries and atomic masses involved Spectroscopic Regions Typical wavelength Approximate energy Spectroscopic region Techniques and Applications (cm) (kcal mole-1) -11 8 10 3 x 10 γ-ray MÖssbauer 10-8 3 x 105 X-ray x-ray diffraction, scattering 10-5 3 x 102 Vacuum UV Electronic Spectra 3 x 10-5 102 Near UV Electronic Spectra 6 x 10-5 5 x 103 Visible Electronic Spectra 10-3 3 x 100 IR Vibrational Spectra 10-2 3 x 10-1 Far IR Vibrational Spectra 10-1 3 x 10-2 Microwave Rotational Spectra 100 3 x 10-3 Microwave Electron paramagnetic resonance 10 3 x 10-4 Radio frequency Nuclear magnetic resonance Adapted from Table 7-1; Biophysical Chemistry, Part II by Cantor and Schimmel Spectroscopic Process • Molecules contain distribution of charges (electrons and nuclei, charges from protons) and spins which is dynamically changed when molecule is exposed to light •In a spectroscopic experiment, light is used to probe a sample. What we seek to understand is: – the RATE at which the molecule responds to this perturbation (this is the response or spectral intensity) – why only certain wavelengths cause changes (this is the spectrum, the wavelength dependence of the response) – the process by which the molecule alters the radiation that emerges from the sample (absorption, scattering, fluorescence, photochemistry, etc.) so we can detect it These tell us about molecular identity, structure, mechanisms and analytical concentrations Magnetic Resonance—different course • Long wavelength radiowaves are of low energy that is sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). -

Rotational Spectroscopy and Interstellar Molecules
Volume -5, Issue-2, April 2015 » Plescia, J. B., and Cintala M. J. (2012), Impact melt Rotational Spectroscopy and Interstellar in small lunar highland craters, J. Geophys. Res., 117, Molecules E00H12,doi:10.1029/2011JE003941 » Plescia J.B. and Spudis P.D. (2014) Impact melt flows at The fact that life exists on Earth is no secret. However, Lowell crater, Planetary and Space Science, 103, 219- understanding the origin of life, its evolution, and the fu- 227 ture of life on Earth remain interesting issues to be ad- » Shkuratov, Y.,Kaydash, V.,Videen, G. (2012)The lunar dressed. That the regions between stars contain by far the crater Giordano Bruno as seen with optical roughness largest reservoir of chemically-bonded matter in nature imagery, Icarus, 218(1), 525-533 obviously demonstrates the importance of chemistry in » Smrekar S. and Pieters C.M. (1985) Near-infrared spec- the interstellar space. The unique detection of over 200 troscopy of probable impact melt from three large lunar different interstellar molecules largely via their rotational highland craters, Icarus, 63, 442-452 spectra has laid to rest the popular perception that the » Srivastava, N., Kumar, D., Gupta, R. P., 2013. Young vastness of space is an empty vacuum dotted with stars, viscous flows in the Lowell crater of Orientale basin, planets, black holes, and other celestial formations. As- Moon: Impact melts or volcanic eruptions? Planetary trochemistry comprises observations, theory and experi- and Space Science, 87, 37-45. ments aimed at understanding the formation of molecules » Stopar J. D., Hawke B. R., Robinson M. S., DeneviB.W., and matter in the Universe i.e. -

Rotational Spectroscopy
Applied Spectroscopy Rotational Spectroscopy Recommended Reading: 1. Banwell and McCash: Chapter 2 2. Atkins: Chapter 16, sections 4 - 8 Aims In this section you will be introduced to 1) Rotational Energy Levels (term values) for diatomic molecules and linear polyatomic molecules 2) The rigid rotor approximation 3) The effects of centrifugal distortion on the energy levels 4) The Principle Moments of Inertia of a molecule. 5) Definitions of symmetric , spherical and asymmetric top molecules. 6) Experimental methods for measuring the pure rotational spectrum of a molecule Microwave Spectroscopy - Rotation of Molecules Microwave Spectroscopy is concerned with transitions between rotational energy levels in molecules. Definition d Electric Dipole: p = q.d +q -q p H Most heteronuclear molecules possess Cl a permanent dipole moment -q +q e.g HCl, NO, CO, H2O... p Molecules can interact with electromagnetic radiation, absorbing or emitting a photon of frequency ω, if they possess an electric dipole moment p, oscillating at the same frequency Gross Selection Rule: A molecule has a rotational spectrum only if it has a permanent dipole moment. Rotating molecule _ _ + + t _ + _ + dipole momentp dipole Homonuclear molecules (e.g. O2, H2, Cl2, Br2…. do not have a permanent dipole moment and therefore do not have a microwave spectrum! General features of rotating systems m Linear velocity v angular velocity v = distance ω = radians O r time time v = ω × r Moment of Inertia I = mr2. A molecule can have three different moments of inertia IA, IB and IC about orthogonal axes a, b and c. 2 I = ∑miri i R Note how ri is defined, it is the perpendicular distance from axis of rotation ri Rigid Diatomic Rotors ro IB = Ic, and IA = 0. -

Photoionization Spectroscopy O
Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, A. Flütsch, C. Romanzin, J.-C. Guillemin, Séverine Boyé-Péronne, B. Gans To cite this version: N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, et al.. Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range. Journal of Molecular Spectroscopy, Elsevier, 2015, 315, pp.206-216. 10.1016/j.jms.2015.03.005. hal-01138635 HAL Id: hal-01138635 https://hal-univ-rennes1.archives-ouvertes.fr/hal-01138635 Submitted on 4 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Photoionization spectroscopy of CH 3C3N in the vacuum-ultraviolet range N. Lamarre a, C. Falvo a, C. Alcaraz b,c, B. Cunha de Miranda b, S. Douin a, A. Fl utsch¨ a, C. Romanzin b, J.-C. Guillemin d, a, a, S. Boy e-P´ eronne´ ∗, B. Gans ∗ aInstitut des Sciences Mol´eculaires d’Orsay, Univ Paris-Sud; CNRS, bat 210, Univ Paris-Sud 91405 Orsay cedex (France) bLaboratoire de Chimie Physique, Univ Paris-Sud; CNRS UMR 8000, bat 350, Univ Paris-Sud 91405 Orsay cedex (France) cSynchrotron SOLEIL, L'Orme des Merisiers, St. -

Low Power Energy Harvesting and Storage Techniques from Ambient Human Powered Energy Sources
University of Northern Iowa UNI ScholarWorks Dissertations and Theses @ UNI Student Work 2008 Low power energy harvesting and storage techniques from ambient human powered energy sources Faruk Yildiz University of Northern Iowa Copyright ©2008 Faruk Yildiz Follow this and additional works at: https://scholarworks.uni.edu/etd Part of the Power and Energy Commons Let us know how access to this document benefits ouy Recommended Citation Yildiz, Faruk, "Low power energy harvesting and storage techniques from ambient human powered energy sources" (2008). Dissertations and Theses @ UNI. 500. https://scholarworks.uni.edu/etd/500 This Open Access Dissertation is brought to you for free and open access by the Student Work at UNI ScholarWorks. It has been accepted for inclusion in Dissertations and Theses @ UNI by an authorized administrator of UNI ScholarWorks. For more information, please contact [email protected]. LOW POWER ENERGY HARVESTING AND STORAGE TECHNIQUES FROM AMBIENT HUMAN POWERED ENERGY SOURCES. A Dissertation Submitted In Partial Fulfillment of the Requirements for the Degree Doctor of Industrial Technology Approved: Dr. Mohammed Fahmy, Chair Dr. Recayi Pecen, Co-Chair Dr. Sue A Joseph, Committee Member Dr. John T. Fecik, Committee Member Dr. Andrew R Gilpin, Committee Member Dr. Ayhan Zora, Committee Member Faruk Yildiz University of Northern Iowa August 2008 UMI Number: 3321009 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Energy Harvesting from Rotating Structures
ENERGY HARVESTING FROM ROTATING STRUCTURES Tzern T. Toh, A. Bansal, G. Hong, Paul D. Mitcheson, Andrew S. Holmes, Eric M. Yeatman Department of Electrical & Electronic Engineering, Imperial College London, U.K. Abstract: In this paper, we analyze and demonstrate a novel rotational energy harvesting generator using gravitational torque. The electro-mechanical behavior of the generator is presented, alongside experimental results from an implementation based on a conventional DC motor. The off-axis performance is also modeled. Designs for adaptive power processing circuitry for optimal power harvesting are presented, using SPICE simulations. Key Words: energy-harvesting, rotational generator, adaptive generator, double pendulum 1. INTRODUCTION with ω the angular rotation rate of the host. Energy harvesting from moving structures has been a topic of much research, particularly for applications in powering wireless sensors [1]. Most motion energy harvesters are inertial, drawing power from the relative motion between an oscillating proof mass and the frame from which it is suspended [2]. For many important applications, including tire pressure sensing and condition monitoring of machinery, the host structure undergoes continuous rotation; in these Fig. 1: Schematic of the gravitational torque cases, previous energy harvesters have typically generator. IA is the armature current and KE is the been driven by the associated vibration. In this motor constant. paper we show that rotational motion can be used directly to harvest power, and that conventional Previously we reported initial experimental rotating machines can be easily adapted to this results for this device, and circuit simulations purpose. based on a buck-boost converter [3]. In this paper All mechanical to electrical transducers rely on we consider a Flyback power conversion circuit, the relative motion of two generator sections. -

Rotational Piezoelectric Energy Harvesting: a Comprehensive Review on Excitation Elements, Designs, and Performances
energies Review Rotational Piezoelectric Energy Harvesting: A Comprehensive Review on Excitation Elements, Designs, and Performances Haider Jaafar Chilabi 1,2,* , Hanim Salleh 3, Waleed Al-Ashtari 4, E. E. Supeni 1,* , Luqman Chuah Abdullah 1 , Azizan B. As’arry 1, Khairil Anas Md Rezali 1 and Mohammad Khairul Azwan 5 1 Department of Mechanical and Manufacturing, Faculty of Engineering, Universiti Putra Malaysia, Serdang 43400, Malaysia; [email protected] (L.C.A.); [email protected] (A.B.A.); [email protected] (K.A.M.R.) 2 Midland Refineries Company (MRC), Ministry of Oil, Baghdad 10022, Iraq 3 Institute of Sustainable Energy, Universiti Tenaga Nasional, Jalan Ikram-Uniten, Kajang 43000, Malaysia; [email protected] 4 Mechanical Engineering Department, College of Engineering University of Baghdad, Baghdad 10022, Iraq; [email protected] 5 Department of Mechanical Engineering, College of Engineering, Universiti Tenaga Nasional, Jalan Ikram-15 Uniten, Kajang 43000, Malaysia; [email protected] * Correspondence: [email protected] (H.J.C.); [email protected] (E.E.S.) Abstract: Rotational Piezoelectric Energy Harvesting (RPZTEH) is widely used due to mechanical rotational input power availability in industrial and natural environments. This paper reviews the recent studies and research in RPZTEH based on its excitation elements and design and their influence on performance. It presents different groups for comparison according to their mechanical Citation: Chilabi, H.J.; Salleh, H.; inputs and applications, such as fluid (air or water) movement, human motion, rotational vehicle tires, Al-Ashtari, W.; Supeni, E.E.; and other rotational operational principal including gears. The work emphasises the discussion of Abdullah, L.C.; As’arry, A.B.; Rezali, K.A.M.; Azwan, M.K. -

Training Fact Sheet - Energy in Autorotations Contact: Nick Mayhew Phone: (321) 567 0386 ______
Training Fact Sheet - Energy in Autorotations Contact: Nick Mayhew Phone: (321) 567 0386 ______________________________________________________________________________________________ Using Energy for Our Benefit these energies cannot be created or destroyed, just transferred from one place to another. Relative Sizes of the Energy There are many ways that these energies inter-relate. Potential energy can be viewed as a source of kinetic (and rotational) energy. It’s interesting to note the relative sizes of these. It’s not easy to compare kinetic and potential energy, as they can be traded for one another. But the relatively small size of the rotational energy is surprising. One DVD on the subject showed that the rotational energy was a very small fraction of the combined kinetic The secret to extracting the maximum flexibility from an and potential energy even at the start of autorotation is to understand the various a typical flare. energies at your disposal. Energy is the ability to do work, and the ones available in an autorotation What makes this relative size difference important is that are: potential, kinetic, and rotational. There is a subtle, the rotor RPM, while the smallest energy, but powerful interplay between these is far and away the most important energy – without the energies that we can use to our benefit – but only if we rotor RPM, it is not possible to control know and understand them. the helicopter and all the other energies are of no use! The process of getting from the time/place of the engine We’ve already identified 3 different stages to the failure to safely on the ground can be autorotation – the descent, the flare and thought of as an exercise in energy management. -

2. Molecular Stucture/Basic Spectroscopy the Electromagnetic Spectrum
2. Molecular stucture/Basic spectroscopy The electromagnetic spectrum Spectral region fooatocadr atomic and molecular spectroscopy E. Hecht (2nd Ed.) Optics, Addison-Wesley Publishing Company,1987 Per-Erik Bengtsson Spectral regions Mo lecu lar spec troscopy o ften dea ls w ith ra dia tion in the ultraviolet (UV), visible, and infrared (IR) spectltral reg ions. • The visible region is from 400 nm – 700 nm • The ultraviolet region is below 400 nm • The infrared region is above 700 nm. 400 nm 500 nm 600 nm 700 nm Spectroscopy: That part of science which uses emission and/or absorption of radiation to deduce atomic/molecular properties Per-Erik Bengtsson Some basics about spectroscopy E = Energy difference = c /c h = Planck's constant, 6.63 10-34 Js ergy nn = Frequency E hn = h/hc /l E = h = hc / c = Velocity of light, 3.0 108 m/s = Wavelength 0 Often the wave number, , is used to express energy. The unit is cm-1. = E / hc = 1/ Example The energy difference between two states in the OH-molecule is 35714 cm-1. Which wavelength is needed to excite the molecule? Answer = 1/ =35714 cm -1 = 1/ = 280 nm. Other ways of expressing this energy: E = hc/ = 656.5 10-19 J E / h = c/ = 9.7 1014 Hz Per-Erik Bengtsson Species in combustion Combustion involves a large number of species Atoms oxygen (O), hydrogen (H), etc. formed by dissociation at high temperatures Diatomic molecules nitrogen (N2), oxygen (O2) carbon monoxide (CO), hydrogen (H2) nitr icoxide (NO), hy droxy l (OH), CH, e tc. -

Hydrogen Conversion in Nanocages
Review Hydrogen Conversion in Nanocages Ernest Ilisca Laboratoire Matériaux et Phénomènes Quantiques, Université de Paris, CNRS, F-75013 Paris, France; [email protected] Abstract: Hydrogen molecules exist in the form of two distinct isomers that can be interconverted by physical catalysis. These ortho and para forms have different thermodynamical properties. Over the last century, the catalysts developed to convert hydrogen from one form to another, in laboratories and industries, were magnetic and the interpretations relied on magnetic dipolar interactions. The variety concentration of a sample and the conversion rates induced by a catalytic action were mostly measured by thermal methods related to the diffusion of the o-p reaction heat. At the turning of the new century, the nature of the studied catalysts and the type of measures and motivations completely changed. Catalysts investigated now are non-magnetic and new spectroscopic measurements have been developed. After a fast survey of the past studies, the review details the spectroscopic methods, emphasizing their originalities, performances and refinements: how Infra-Red measurements charac- terize the catalytic sites and follow the conversion in real-time, Ultra-Violet irradiations explore the electronic nature of the reaction and hyper-frequencies driving the nuclear spins. The new catalysts, metallic or insulating, are detailed to display the operating electronic structure. New electromagnetic mechanisms, involving energy and momenta transfers, are discovered providing a classification frame for the newly observed reactions. Keywords: molecular spectroscopy; nanocages; electronic excitations; nuclear magnetism Citation: Ilisca, E. Hydrogen Conversion in Nanocages. Hydrogen The intertwining of quantum, spectroscopic and thermodynamical properties of the 2021, 2, 160–206.