Rotational Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vibrational-Rotational Spectroscopy ( )
Vibrational-Rotational Spectroscopy Vibrational-Rotational Spectrum of Heteronuclear Diatomic Absorption of mid-infrared light (~300-4000 cm-1): • Molecules can change vibrational and rotational states • Typically at room temperature, only ground vibrational state populated but several rotational levels may be populated. • Treating as harmonic oscillator and rigid rotor: subject to selection rules ∆v = ±1 and ∆J = ±1 EEEfield=∆ vib +∆ rot =ω =−EEfi = EvJEvJ()′,, ′ −( ′′ ′′ ) ω 11 vvvBJJvvBJJ==00()′′′′′′′′′ +++−++()11() () + 2πc 22 At room temperature, typically v=0′′ and ∆v = +1: ′ ′ ′′ ′′ vv=+0 BJJ() +−11 JJ( +) Now, since higher lying rotational levels can be populated, we can have: ∆=+J 1 JJ′′′=+1 vv= ++21 BJ( ′′ ) R − branch 0 P− branch ∆=−J 1 JJ′′′=−1 vv=−0 2 BJ′′ J’=4 J’=3 J’=1 v’=1 J’=0 P branch Q branch J’’=3 12B J’’=2 6B J’’=1 2B v’’=0 J’’=0 EJ” =0 2B 2B 4B 2B 2B 2B -6B -4B -2B +2B +4B +8B ν ν 0 By measuring absorption splittings, we can get B . From that, the bond length! In polyatomics, we can also have a Q branch, where ∆J0= and all transitions lie at ν=ν0 . This transition is allowed for perpendicular bands: ∂µ ∂q ⊥ to molecular symmetry axis. Intensity of Vibrational-Rotational Transitions There is generally no thermal population in upper (final) state (v’,J’) so intensity should scale as population of lower J state (J”). ∆=NNvJNvJ(,′ ′′′′′′′ ) − (, ) ≈ NJ ( ) NJ()′′∝− gJ ()exp( ′′ EJ′′ / kT ) =+()21exp(J′′ −hcBJ ′′() J ′′ + 1/) kT 5.33 Lecture Notes: Vibrational-Rotational Spectroscopy Page 2 Rotational Populations at Room Temperature for B = 5 cm-1 gJ'' thermal population NJ'' 0 5 10 15 20 Rotational Quantum Number J'' So, the vibrational-rotational spectrum should look like equally spaced lines about ν 0 with sidebands peaked at J’’>0. -
Optical Spectroscopy - Processes Monitored UV/ Fluorescence/ IR/ Raman/ Circular Dichroism
Time out—states and transitions Spectroscopy—transitions between energy states of a molecule excited by absorption or emission of a photon hν = ∆E = Ei -Ef Energy levels due to interactions between parts of molecule (atoms, electrons and nucleii) as described by quantum mechanics, and are characteristic of components involved, i.e. electron distributions (orbitals), bond strengths and types plus molecular geometries and atomic masses involved Spectroscopic Regions Typical wavelength Approximate energy Spectroscopic region Techniques and Applications (cm) (kcal mole-1) -11 8 10 3 x 10 γ-ray MÖssbauer 10-8 3 x 105 X-ray x-ray diffraction, scattering 10-5 3 x 102 Vacuum UV Electronic Spectra 3 x 10-5 102 Near UV Electronic Spectra 6 x 10-5 5 x 103 Visible Electronic Spectra 10-3 3 x 100 IR Vibrational Spectra 10-2 3 x 10-1 Far IR Vibrational Spectra 10-1 3 x 10-2 Microwave Rotational Spectra 100 3 x 10-3 Microwave Electron paramagnetic resonance 10 3 x 10-4 Radio frequency Nuclear magnetic resonance Adapted from Table 7-1; Biophysical Chemistry, Part II by Cantor and Schimmel Spectroscopic Process • Molecules contain distribution of charges (electrons and nuclei, charges from protons) and spins which is dynamically changed when molecule is exposed to light •In a spectroscopic experiment, light is used to probe a sample. What we seek to understand is: – the RATE at which the molecule responds to this perturbation (this is the response or spectral intensity) – why only certain wavelengths cause changes (this is the spectrum, the wavelength dependence of the response) – the process by which the molecule alters the radiation that emerges from the sample (absorption, scattering, fluorescence, photochemistry, etc.) so we can detect it These tell us about molecular identity, structure, mechanisms and analytical concentrations Magnetic Resonance—different course • Long wavelength radiowaves are of low energy that is sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). -

Rotational Spectroscopy and Interstellar Molecules
Volume -5, Issue-2, April 2015 » Plescia, J. B., and Cintala M. J. (2012), Impact melt Rotational Spectroscopy and Interstellar in small lunar highland craters, J. Geophys. Res., 117, Molecules E00H12,doi:10.1029/2011JE003941 » Plescia J.B. and Spudis P.D. (2014) Impact melt flows at The fact that life exists on Earth is no secret. However, Lowell crater, Planetary and Space Science, 103, 219- understanding the origin of life, its evolution, and the fu- 227 ture of life on Earth remain interesting issues to be ad- » Shkuratov, Y.,Kaydash, V.,Videen, G. (2012)The lunar dressed. That the regions between stars contain by far the crater Giordano Bruno as seen with optical roughness largest reservoir of chemically-bonded matter in nature imagery, Icarus, 218(1), 525-533 obviously demonstrates the importance of chemistry in » Smrekar S. and Pieters C.M. (1985) Near-infrared spec- the interstellar space. The unique detection of over 200 troscopy of probable impact melt from three large lunar different interstellar molecules largely via their rotational highland craters, Icarus, 63, 442-452 spectra has laid to rest the popular perception that the » Srivastava, N., Kumar, D., Gupta, R. P., 2013. Young vastness of space is an empty vacuum dotted with stars, viscous flows in the Lowell crater of Orientale basin, planets, black holes, and other celestial formations. As- Moon: Impact melts or volcanic eruptions? Planetary trochemistry comprises observations, theory and experi- and Space Science, 87, 37-45. ments aimed at understanding the formation of molecules » Stopar J. D., Hawke B. R., Robinson M. S., DeneviB.W., and matter in the Universe i.e. -

ROTATIONAL SPECTRA (Microwave Spectroscopy)
UNIT-1 ROTATIONAL SPECTRA (Microwave Spectroscopy) Lesson Structure 1.0 Objective 1.1 Introduction 1.2 Classification of molecules 1.3 Rotational spectra of regid diatomic molecules 1.4 Selection rules 1.5 Non-rigid rotator 1.6 Spectrum of a non-rigid rotator 1.7 Linear polyatomic molecules 1.8 Non-linear polyatomic molecules 1.9 Asymmetric top molecles 1.10. Starck effect Solved Problems Model Questions References 1.0 OBJECIVES After studyng this unit, you should be able to • Define the monent of inertia • Discuss the rotational spectra of rigid linear diatomic molecule • Spectrum of non-rigid rotator • Moment of inertia of linear polyatomic molecules Rotational Spectra (Microwave Spectroscopy) • Explain the effect of isotopic substitution and non-rigidity on the rotational spectra of a molecule. • Classify various molecules according to thier values of moment of inertia • Know the selection rule for a rigid diatomic molecule. 1.0 INTRODUCTION Spectroscopy in the microwave region is concerned with the study of pure rotational motion of molecules. The condition for a molecule to be microwave active is that the molecule must possess a permanent dipole moment, for example, HCl, CO etc. The rotating dipole then generates an electric field which may interact with the electrical component of the microwave radiation. Rotational spectra are obtained when the energy absorbed by the molecule is so low that it can cause transition only from one rotational level to another within the same vibrational level. Microwave spectroscopy is a useful technique and gives the values of molecular parameters such as bond lengths, dipole moments and nuclear spins etc. -

Particle-On-A-Ring” Suppose a Diatomic Molecule Rotates in Such a Way That the Vibration of the Bond Is Unaffected by the Rotation
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015 Lecture 17 2/25/15 Recommended Reading Atkins & DePaula 9.5 A. Background in Rotational Mechanics Two masses m1 and m2 connected by a weightless rigid “bond” of length r rotates with angular velocity rv where v is the linear velocity. As with vibrations we can render this rotation motion in simpler form by fixing one end of the bond to the origin terminating the other end with reduced mass , and allowing the reduced mass to rotate freely For two masses m1 and m2 separated by r the center of mass (CoM) is defined by the condition mr11 mr 2 2 (17.1) where m2,1 rr1,2 mm12 (17.2) Figure 17.1: Location of center of mass (CoM0 for two masses separated by a distance r. The moment of inertia I plays the role in the rotational energy that is played by mass in translational energy. The moment of inertia is 22 I mr11 mr 2 2 (17.3) Putting 17.3 into 17.4 we obtain I r 2 (17.4) mm where 12 . mm12 We can express the rotational kinetic energy K in terms of the angular momentum. The angular momentum is defined in terms of the cross product between the position vector r and the linear momentum vector p: Lrp (17.5) prprvrsin where the angle between p and r is ninety degrees for circular motion. As a cross product, the angular momentum vector L is perpendicular to the plane defined by the r and p vectors as shown in Figure 17.2 We can use equation 17.6 to obtain an expression for the kinetic energy in terms of the angular momentum L: Figure 17.2: The angular momentum L is a cross product of the position r vector and the linear momentum p=mv vector. -

Rotational Spectroscopy
Applied Spectroscopy Rotational Spectroscopy Recommended Reading: 1. Banwell and McCash: Chapter 2 2. Atkins: Chapter 16, sections 4 - 8 Aims In this section you will be introduced to 1) Rotational Energy Levels (term values) for diatomic molecules and linear polyatomic molecules 2) The rigid rotor approximation 3) The effects of centrifugal distortion on the energy levels 4) The Principle Moments of Inertia of a molecule. 5) Definitions of symmetric , spherical and asymmetric top molecules. 6) Experimental methods for measuring the pure rotational spectrum of a molecule Microwave Spectroscopy - Rotation of Molecules Microwave Spectroscopy is concerned with transitions between rotational energy levels in molecules. Definition d Electric Dipole: p = q.d +q -q p H Most heteronuclear molecules possess Cl a permanent dipole moment -q +q e.g HCl, NO, CO, H2O... p Molecules can interact with electromagnetic radiation, absorbing or emitting a photon of frequency ω, if they possess an electric dipole moment p, oscillating at the same frequency Gross Selection Rule: A molecule has a rotational spectrum only if it has a permanent dipole moment. Rotating molecule _ _ + + t _ + _ + dipole momentp dipole Homonuclear molecules (e.g. O2, H2, Cl2, Br2…. do not have a permanent dipole moment and therefore do not have a microwave spectrum! General features of rotating systems m Linear velocity v angular velocity v = distance ω = radians O r time time v = ω × r Moment of Inertia I = mr2. A molecule can have three different moments of inertia IA, IB and IC about orthogonal axes a, b and c. 2 I = ∑miri i R Note how ri is defined, it is the perpendicular distance from axis of rotation ri Rigid Diatomic Rotors ro IB = Ic, and IA = 0. -

Photoionization Spectroscopy O
Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, A. Flütsch, C. Romanzin, J.-C. Guillemin, Séverine Boyé-Péronne, B. Gans To cite this version: N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, et al.. Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range. Journal of Molecular Spectroscopy, Elsevier, 2015, 315, pp.206-216. 10.1016/j.jms.2015.03.005. hal-01138635 HAL Id: hal-01138635 https://hal-univ-rennes1.archives-ouvertes.fr/hal-01138635 Submitted on 4 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Photoionization spectroscopy of CH 3C3N in the vacuum-ultraviolet range N. Lamarre a, C. Falvo a, C. Alcaraz b,c, B. Cunha de Miranda b, S. Douin a, A. Fl utsch¨ a, C. Romanzin b, J.-C. Guillemin d, a, a, S. Boy e-P´ eronne´ ∗, B. Gans ∗ aInstitut des Sciences Mol´eculaires d’Orsay, Univ Paris-Sud; CNRS, bat 210, Univ Paris-Sud 91405 Orsay cedex (France) bLaboratoire de Chimie Physique, Univ Paris-Sud; CNRS UMR 8000, bat 350, Univ Paris-Sud 91405 Orsay cedex (France) cSynchrotron SOLEIL, L'Orme des Merisiers, St. -

Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum
Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum We can now extend the Rigid Rotor problem to a rotation in 3D, corre- sponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordi- nates given as ∂2 ∂2 ∂2 ∂2 2 ∂ 1 1 ∂2 1 ∂ ∂ ∇2 = + + = + + + sin θ ∂x2 ∂y2 ∂z2 ∂r2 r ∂r r2 sin2 θ ∂φ2 sin θ ∂θ ∂θ For constant radius the first two terms are zero and we have 2 2 1 ∂2 1 ∂ ∂ Hˆ (θ, φ) = − ~ ∇2 = − ~ + sin θ 2m 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ We also note that Lˆ2 Hˆ = 2I where the operator for squared angular momentum is given by 1 ∂2 1 ∂ ∂ Lˆ2 = − 2 + sin θ ~ sin2 θ ∂φ2 sin θ ∂θ ∂θ The Schr¨odingerequation is given by 2 1 ∂2ψ(θ, φ) 1 ∂ ∂ψ(θ, φ) − ~ + sin θ = Eψ(θ, φ) 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ The wavefunctions are quantized in 2 directions corresponding to θ and φ. It is possible to derive the solutions, but we will not do it here. The solutions are denoted by Yl,ml (θ, φ) and are called spherical harmonics. The quantum 1 numbers take values l = 0, 1, 2, 3, .... and ml = 0, ±1, ±2, ... ± l. The energy depends only on l and is given by 2 E = l(l + 1) ~ 2I The first few spherical harmonics are given by r 1 Y = 0,0 4π r 3 Y = cos(θ) 1,0 4π r 3 Y = sin(θ)e±iφ 1,±1 8π r 5 Y = (3 cos2 θ − 1) 2,0 16π r 15 Y = cos θ sin θe±iφ 2,±1 8π r 15 Y = sin2 θe±2iφ 2,±2 32π These spherical harmonics are related to atomic orbitals in the H-atom. -

A 3D Model of the Rigid Rotor Supported by Journal Bearings)
A 3D MODEL OF THE RIGID ROTOR SUPPORTED BY JOURNAL BEARINGS) Jiří TŮMA1, Radim KLEČKA2, Jaromír ŠKUTA3 and Jiří ŠIMEK4 1 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 2 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 3 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 4 Techlab s.r.o., Sokolovská 207, 190 00 Praha 9, [email protected] Keywords: up to 7 keywords (10 pt) 1. Introduction It is known that the journal bearing with an oil film becomes instable if the rotor rotation speed crosses a certain value, which is called the Bently-Muszynska threshold [1]. To prevent the rotor instability, the active control can be employed. The arrangement of proximity probes and piezoactuators in a rotor system is shown in figure 1. It is assumed that the bushings (carrier ring), inserted into pedestals with clearance, is a movable part in two perpendicular directions while rotor is rotating. Im(r) Bushing Proximity probes Re(r) Ω Re(u) Journal Piezoactuators Im(r) Fig. 1. Journal coordinates and arrangement The research work supported by the GAČR (project no. 101/07/1345) is aimed at the design of the journal bearing active control based on the carrier ring position manipulation by the piezoactuators according to the proximity probe signals, which are a part of the closed loop including a controller. The effect of the feedback on the rotor stability is analyzed by [5]. The test stand of the TECHLAB design [1] is shown in figure 2. -

Hydrogen Conversion in Nanocages
Review Hydrogen Conversion in Nanocages Ernest Ilisca Laboratoire Matériaux et Phénomènes Quantiques, Université de Paris, CNRS, F-75013 Paris, France; [email protected] Abstract: Hydrogen molecules exist in the form of two distinct isomers that can be interconverted by physical catalysis. These ortho and para forms have different thermodynamical properties. Over the last century, the catalysts developed to convert hydrogen from one form to another, in laboratories and industries, were magnetic and the interpretations relied on magnetic dipolar interactions. The variety concentration of a sample and the conversion rates induced by a catalytic action were mostly measured by thermal methods related to the diffusion of the o-p reaction heat. At the turning of the new century, the nature of the studied catalysts and the type of measures and motivations completely changed. Catalysts investigated now are non-magnetic and new spectroscopic measurements have been developed. After a fast survey of the past studies, the review details the spectroscopic methods, emphasizing their originalities, performances and refinements: how Infra-Red measurements charac- terize the catalytic sites and follow the conversion in real-time, Ultra-Violet irradiations explore the electronic nature of the reaction and hyper-frequencies driving the nuclear spins. The new catalysts, metallic or insulating, are detailed to display the operating electronic structure. New electromagnetic mechanisms, involving energy and momenta transfers, are discovered providing a classification frame for the newly observed reactions. Keywords: molecular spectroscopy; nanocages; electronic excitations; nuclear magnetism Citation: Ilisca, E. Hydrogen Conversion in Nanocages. Hydrogen The intertwining of quantum, spectroscopic and thermodynamical properties of the 2021, 2, 160–206. -
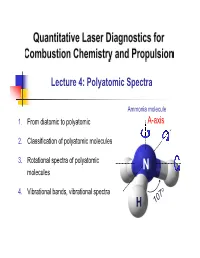
Lecture 4: Polyatomic Spectra
Lecture 4: Polyatomic Spectra Ammonia molecule 1. From diatomic to polyatomic A-axis 2. Classification of polyatomic molecules 3. Rotational spectra of polyatomic N molecules 4. Vibrational bands, vibrational spectra H 1. From diatomic to polyatomic Rotation – Diatomics Recall: For diatomic molecules Energy: FJ ,cm1 BJJ 1 DJ 2 J 12 R.R. Centrifugal distortion constant h Rotational constant: B,cm1 8 2 Ic Selection Rule: J ' J"1 J 1 3 Line position: J "1J " 2BJ"1 4D J"1 Notes: 2 1. D is small, i.e., D / B 4B / vib 1 2 2 D B 1.7 6 2. E.g., for NO, 4 4 310 B NO e 1900 → Even @ J=60, D / B J 2 ~ 0.01 What about polyatomics (≥3 atoms)? 2 1. From diatomic to polyatomic 3D-body rotation B . Convention: A A-axis is the “unique” or “figure” axis, along which lies the molecule’s C defining symmetry . 3 principal axes (orthogonal): A, B, C . 3 principal moments of inertia: IA, IB, IC . Molecules are classified in terms of the relative values of IA, IB, IC 3 2. Classification of polyatomic molecules Types of molecules Linear Symmetric Asymmetric Type Spherical Tops Molecules Tops Rotors Relative IB=IC≠IA magnitudes IB=IC; IA≈0* IA=IB=IC IA≠IB≠IC IA≠0 of IA,B,C NH CO2 3 H2O CH4 C2H2 CH F Examples 3 Acetylene NO2 OCS BCl3 Carbon Boron oxysulfide trichloride Relatively simple No dipole moment Largest category Not microwave active Most complex *Actually finite, but quantized momentum means it is in lowest state of rotation 4 2. -

Molecular Energy Levels
MOLECULAR ENERGY LEVELS DR IMRANA ASHRAF OUTLINE q MOLECULE q MOLECULAR ORBITAL THEORY q MOLECULAR TRANSITIONS q INTERACTION OF RADIATION WITH MATTER q TYPES OF MOLECULAR ENERGY LEVELS q MOLECULE q In nature there exist 92 different elements that correspond to stable atoms. q These atoms can form larger entities- called molecules. q The number of atoms in a molecule vary from two - as in N2 - to many thousand as in DNA, protiens etc. q Molecules form when the total energy of the electrons is lower in the molecule than in individual atoms. q The reason comes from the Aufbau principle - to put electrons into the lowest energy configuration in atoms. q The same principle goes for molecules. q MOLECULE q Properties of molecules depend on: § The specific kind of atoms they are composed of. § The spatial structure of the molecules - the way in which the atoms are arranged within the molecule. § The binding energy of atoms or atomic groups in the molecule. TYPES OF MOLECULES q MONOATOMIC MOLECULES § The elements that do not have tendency to form molecules. § Elements which are stable single atom molecules are the noble gases : helium, neon, argon, krypton, xenon and radon. q DIATOMIC MOLECULES § Diatomic molecules are composed of only two atoms - of the same or different elements. § Examples: hydrogen (H2), oxygen (O2), carbon monoxide (CO), nitric oxide (NO) q POLYATOMIC MOLECULES § Polyatomic molecules consist of a stable system comprising three or more atoms. TYPES OF MOLECULES q Empirical, Molecular And Structural Formulas q Empirical formula: Indicates the simplest whole number ratio of all the atoms in a molecule.