S18 Ch. 5 Rigid Rotator
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Particle-On-A-Ring” Suppose a Diatomic Molecule Rotates in Such a Way That the Vibration of the Bond Is Unaffected by the Rotation
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015 Lecture 17 2/25/15 Recommended Reading Atkins & DePaula 9.5 A. Background in Rotational Mechanics Two masses m1 and m2 connected by a weightless rigid “bond” of length r rotates with angular velocity rv where v is the linear velocity. As with vibrations we can render this rotation motion in simpler form by fixing one end of the bond to the origin terminating the other end with reduced mass , and allowing the reduced mass to rotate freely For two masses m1 and m2 separated by r the center of mass (CoM) is defined by the condition mr11 mr 2 2 (17.1) where m2,1 rr1,2 mm12 (17.2) Figure 17.1: Location of center of mass (CoM0 for two masses separated by a distance r. The moment of inertia I plays the role in the rotational energy that is played by mass in translational energy. The moment of inertia is 22 I mr11 mr 2 2 (17.3) Putting 17.3 into 17.4 we obtain I r 2 (17.4) mm where 12 . mm12 We can express the rotational kinetic energy K in terms of the angular momentum. The angular momentum is defined in terms of the cross product between the position vector r and the linear momentum vector p: Lrp (17.5) prprvrsin where the angle between p and r is ninety degrees for circular motion. As a cross product, the angular momentum vector L is perpendicular to the plane defined by the r and p vectors as shown in Figure 17.2 We can use equation 17.6 to obtain an expression for the kinetic energy in terms of the angular momentum L: Figure 17.2: The angular momentum L is a cross product of the position r vector and the linear momentum p=mv vector. -

Rotational Spectroscopy
Applied Spectroscopy Rotational Spectroscopy Recommended Reading: 1. Banwell and McCash: Chapter 2 2. Atkins: Chapter 16, sections 4 - 8 Aims In this section you will be introduced to 1) Rotational Energy Levels (term values) for diatomic molecules and linear polyatomic molecules 2) The rigid rotor approximation 3) The effects of centrifugal distortion on the energy levels 4) The Principle Moments of Inertia of a molecule. 5) Definitions of symmetric , spherical and asymmetric top molecules. 6) Experimental methods for measuring the pure rotational spectrum of a molecule Microwave Spectroscopy - Rotation of Molecules Microwave Spectroscopy is concerned with transitions between rotational energy levels in molecules. Definition d Electric Dipole: p = q.d +q -q p H Most heteronuclear molecules possess Cl a permanent dipole moment -q +q e.g HCl, NO, CO, H2O... p Molecules can interact with electromagnetic radiation, absorbing or emitting a photon of frequency ω, if they possess an electric dipole moment p, oscillating at the same frequency Gross Selection Rule: A molecule has a rotational spectrum only if it has a permanent dipole moment. Rotating molecule _ _ + + t _ + _ + dipole momentp dipole Homonuclear molecules (e.g. O2, H2, Cl2, Br2…. do not have a permanent dipole moment and therefore do not have a microwave spectrum! General features of rotating systems m Linear velocity v angular velocity v = distance ω = radians O r time time v = ω × r Moment of Inertia I = mr2. A molecule can have three different moments of inertia IA, IB and IC about orthogonal axes a, b and c. 2 I = ∑miri i R Note how ri is defined, it is the perpendicular distance from axis of rotation ri Rigid Diatomic Rotors ro IB = Ic, and IA = 0. -

Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum
Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum We can now extend the Rigid Rotor problem to a rotation in 3D, corre- sponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordi- nates given as ∂2 ∂2 ∂2 ∂2 2 ∂ 1 1 ∂2 1 ∂ ∂ ∇2 = + + = + + + sin θ ∂x2 ∂y2 ∂z2 ∂r2 r ∂r r2 sin2 θ ∂φ2 sin θ ∂θ ∂θ For constant radius the first two terms are zero and we have 2 2 1 ∂2 1 ∂ ∂ Hˆ (θ, φ) = − ~ ∇2 = − ~ + sin θ 2m 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ We also note that Lˆ2 Hˆ = 2I where the operator for squared angular momentum is given by 1 ∂2 1 ∂ ∂ Lˆ2 = − 2 + sin θ ~ sin2 θ ∂φ2 sin θ ∂θ ∂θ The Schr¨odingerequation is given by 2 1 ∂2ψ(θ, φ) 1 ∂ ∂ψ(θ, φ) − ~ + sin θ = Eψ(θ, φ) 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ The wavefunctions are quantized in 2 directions corresponding to θ and φ. It is possible to derive the solutions, but we will not do it here. The solutions are denoted by Yl,ml (θ, φ) and are called spherical harmonics. The quantum 1 numbers take values l = 0, 1, 2, 3, .... and ml = 0, ±1, ±2, ... ± l. The energy depends only on l and is given by 2 E = l(l + 1) ~ 2I The first few spherical harmonics are given by r 1 Y = 0,0 4π r 3 Y = cos(θ) 1,0 4π r 3 Y = sin(θ)e±iφ 1,±1 8π r 5 Y = (3 cos2 θ − 1) 2,0 16π r 15 Y = cos θ sin θe±iφ 2,±1 8π r 15 Y = sin2 θe±2iφ 2,±2 32π These spherical harmonics are related to atomic orbitals in the H-atom. -

A 3D Model of the Rigid Rotor Supported by Journal Bearings)
A 3D MODEL OF THE RIGID ROTOR SUPPORTED BY JOURNAL BEARINGS) Jiří TŮMA1, Radim KLEČKA2, Jaromír ŠKUTA3 and Jiří ŠIMEK4 1 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 2 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 3 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 4 Techlab s.r.o., Sokolovská 207, 190 00 Praha 9, [email protected] Keywords: up to 7 keywords (10 pt) 1. Introduction It is known that the journal bearing with an oil film becomes instable if the rotor rotation speed crosses a certain value, which is called the Bently-Muszynska threshold [1]. To prevent the rotor instability, the active control can be employed. The arrangement of proximity probes and piezoactuators in a rotor system is shown in figure 1. It is assumed that the bushings (carrier ring), inserted into pedestals with clearance, is a movable part in two perpendicular directions while rotor is rotating. Im(r) Bushing Proximity probes Re(r) Ω Re(u) Journal Piezoactuators Im(r) Fig. 1. Journal coordinates and arrangement The research work supported by the GAČR (project no. 101/07/1345) is aimed at the design of the journal bearing active control based on the carrier ring position manipulation by the piezoactuators according to the proximity probe signals, which are a part of the closed loop including a controller. The effect of the feedback on the rotor stability is analyzed by [5]. The test stand of the TECHLAB design [1] is shown in figure 2. -
Rotational Spectroscopy
Rotational spectroscopy - Involve transitions between rotational states of the molecules (gaseous state!) - Energy difference between rotational levels of molecules has the same order of magnitude with microwave energy - Rotational spectroscopy is called pure rotational spectroscopy, to distinguish it from roto-vibrational spectroscopy (the molecule changes its state of vibration and rotation simultaneously) and vibronic spectroscopy (the molecule changes its electronic state and vibrational state simultaneously) Molecules do not rotate around an arbitrary axis! Generally, the rotation is around the mass center of the molecule. The rotational axis must allow the conservation of M R α pα const kinetic angular momentum. α Rotational spectroscopy Rotation of diatomic molecule - Classical description Diatomic molecule = a system formed by 2 different masses linked together with a rigid connector (rigid rotor = the bond length is assumed to be fixed!). The system rotation around the mass center is equivalent with the rotation of a particle with the mass μ (reduced mass) around the center of mass. 2 2 2 2 m1m2 2 The moment of inertia: I miri m1r1 m2r2 R R i m1 m2 Moment of inertia (I) is the rotational equivalent of mass (m). Angular velocity () is the equivalent of linear velocity (v). Er → rotational kinetic energy L = I → angular momentum mv 2 p2 Iω2 L2 E E c 2 2m r 2 2I Quantum rotation: The diatomic rigid rotor The rigid rotor represents the quantum mechanical “particle on a sphere” problem: Rotational energy is purely -
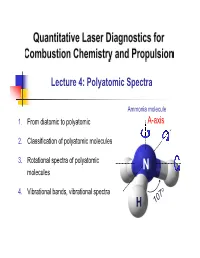
Lecture 4: Polyatomic Spectra
Lecture 4: Polyatomic Spectra Ammonia molecule 1. From diatomic to polyatomic A-axis 2. Classification of polyatomic molecules 3. Rotational spectra of polyatomic N molecules 4. Vibrational bands, vibrational spectra H 1. From diatomic to polyatomic Rotation – Diatomics Recall: For diatomic molecules Energy: FJ ,cm1 BJJ 1 DJ 2 J 12 R.R. Centrifugal distortion constant h Rotational constant: B,cm1 8 2 Ic Selection Rule: J ' J"1 J 1 3 Line position: J "1J " 2BJ"1 4D J"1 Notes: 2 1. D is small, i.e., D / B 4B / vib 1 2 2 D B 1.7 6 2. E.g., for NO, 4 4 310 B NO e 1900 → Even @ J=60, D / B J 2 ~ 0.01 What about polyatomics (≥3 atoms)? 2 1. From diatomic to polyatomic 3D-body rotation B . Convention: A A-axis is the “unique” or “figure” axis, along which lies the molecule’s C defining symmetry . 3 principal axes (orthogonal): A, B, C . 3 principal moments of inertia: IA, IB, IC . Molecules are classified in terms of the relative values of IA, IB, IC 3 2. Classification of polyatomic molecules Types of molecules Linear Symmetric Asymmetric Type Spherical Tops Molecules Tops Rotors Relative IB=IC≠IA magnitudes IB=IC; IA≈0* IA=IB=IC IA≠IB≠IC IA≠0 of IA,B,C NH CO2 3 H2O CH4 C2H2 CH F Examples 3 Acetylene NO2 OCS BCl3 Carbon Boron oxysulfide trichloride Relatively simple No dipole moment Largest category Not microwave active Most complex *Actually finite, but quantized momentum means it is in lowest state of rotation 4 2. -

Quantum Control of Molecular Rotation Is Challenging and Promising at the Same Time
Quantum control of molecular rotation Christiane P. Koch,∗ Mikhail Lemeshko,† Dominique Sugny‡ October 10, 2019 Abstract The angular momentum of molecules, or, equivalently, their rotation in three-dimensional space, is ideally suited for quantum control. Molecular angular momentum is naturally quantized, time evolution is governed by a well-known Hamiltonian with only a few accurately known parameters, and transitions between rota- tional levels can be driven by external fields from various parts of the electromagnetic spectrum. Control over the rotational motion can be exerted in one-, two- and many-body scenarios, thereby allowing to probe Anderson localization, target stereoselectivity of bimolecular reactions, or encode quantum information, to name just a few examples. The corresponding approaches to quantum control are pursued within sepa- rate, and typically disjoint, subfields of physics, including ultrafast science, cold collisions, ultracold gases, quantum information science, and condensed matter physics. It is the purpose of this review to present the various control phenomena, which all rely on the same underlying physics, within a unified framework. To this end, we recall the Hamiltonian for free rotations, assuming the rigid rotor approximation to be valid, and summarize the different ways for a rotor to interact with external electromagnetic fields. These inter- actions can be exploited for control — from achieving alignment, orientation, or laser cooling in a one-body framework, steering bimolecular collisions, or realizing a quantum computer or quantum simulator in the many-body setting. 1 Introduction Molecules, unlike atoms, are extended objects that possess a number of different types of motion. In particular, the geometric arrangement of their constituent atoms endows molecules with the basic capability to rotate in three-dimensional space. -

Chapter 7. a Quantum Mechanical Model for the Vibration and Rotation of Molecules
Chapter 7. A Quantum Mechanical Model for the Vibration and Rotation of Molecules Harmonic oscillator: Hooke’s law: F kx, x is displacement Harmonic potential: 1 2 V (x) Fdx kx 2 k is force constant: d 2V k (curvature of V at equilibrium) 2 dx x0 Newton’s equation: d 2x m F kx (diff. eqn) dt 2 Solutions: x = Asint, (position) 1/ 2 (k/m) (vibrational frequency) 1 Verify: d 2 Asin t d lhs m mA cost mA2 sin t dt 2 dt k m Asin t kx rhs m Momentum: dx p m mAcost dt Energy: p2 E V 2m (mAcost)2 1 k(Asin t)2 2m 2 m 2 A2 (cost)2 (sin t)2 2 m 2 A2 kA2 2 2 When t = 0, x = 0 and p = p = mA max When t = , p = 0 and x = x = A max Energy conservation is maintained by oscillation between kinetic and potential energies. 2 Schrödinger equation for harmonic oscillator: 2 2 d 1 2 2 kx E 2m dx 2 Energy is quantized: 1 E 0, 1, ... 2 where the vibrational frequency 1/ 2 k m Restriction of motion leads to uncertainty in x and p, and quantization of energy. Wavefunctions: y2 / 2 (x) N H (y)e where 1/ 4 mk y x, 2 Normalization factor 3 1/ 2 N 2! H (y) is the Hermite polynomial H0 (y) 1 H1(y) 2y 2 H2 (y) 4y 2 ... H1 2yH 2H1 (recursion relation) Let’s verify for the ground state 2 x2 / 2 0 (x) N0e 2 2 d 1 2 2 x 2 / 2 lhs N0 2 kx e 2m dx 2 2 d 2 x 2 / 2 2 1 2 2 x 2 / 2 N0 e x kx e 2m dx 2 2 2 x 2 / 2 2 2 2 x 2 / 2 2 1 2 2 x 2 / 2 N0 e x e kx e 2m 2 2 4 2 2 k 2 2 2 x / 2 N0 x e 2 2m 2m Note that 4 1/ 4 mk 2 It is not difficult to prove that the first term is zero, and the second term as / 2. -

5.61 F17 Lecture 17: Rigid Rotor I
5.61 Fall 2017 Lecture #17 Page 1 5.61 Lecture #17 Rigid Rotor I Read McQuarrie: Chapters E and 6. Rigid Rotors | molecular rotation and the universal angular part of all central force problems { another exactly solved problem. The rotor is free, thus Hb = Tb (V = 0). Once again, we are more interested in: D E * EJ and Jb t 2 * effects of Jb , Jbz, Jb± = Jbx ± iJby (\raising" and \lowering" or \ladder" operators) * qualitative stuff about shape (nodal surfaces) of JM (θ; φ) than we are in the actual form of JM (θ; φ) and in the method for solving the rigid rotor Schr¨odingerEquation. Next Lecture { no differential equation, no , just: P *[ Ji; Jj] = i} k "ijkJk definition of an angular momentum operator 2 2 * J JM = } J(J + 1) JM * Jz JM = }M JM * J± = Jx ± iJy 1=2 * J± JM = [J(J + 1) − M(M ± 1)] JM±1 The standard problem is for the motion of a mass point constrained to the surface of a sphere. However, we are really interested in the rotational motion of a rigid diatomic molecule. Mass point is the same as one end of a rotor (length r0, mass µ) with bond axis extending from the coordinate origin at the Center of Mass. 5.61 Fall 2017 Lecture #17 Page 2 Z M is projection of J on Z J M θ J m1 r1 rcm r2 m2 Y X at origin, J \precesses" about Z. Why r0 = r1 + r2 do we say this when there is no motion in solutions to TISE? For a molecule, there are two coordinate systems: the body–fixed system denoted by x; y; z and the laboratory-fixed system denoted by X; Y; Z. -
Lecture 2: Rotational and Vibrational Spectra
Lecture 2: Rotational and Vibrational Spectra 1. Light-matter interaction 2. Rigid-rotor model for diatomic molecule 3. Non-rigid rotation 4. Vibration-rotation for diatomics 1. Light-matter interaction Possibilities of interaction Permanent electric dipole moment Rotation and vibration produce oscillating dipole (Emission/Absorption) H2O HCl Energy ∆E Absorption = qd Emission What if Homonuclear? Induced polarization Elastic scattering (Raman scattering) (Rayleigh scattering) Virtual State vs s < m or as Inelastic scattering as > 2 1. Light-matter interaction • Line position () is determined by Elements of spectra: difference between energy levels Line position • What determines the energy levels? Line strength • Quantum Mechanics! Line shapes Rotation: Microwave Region (∆J) Internal Energy: Electric dipole moment: qi r i E = E (n)+E ()+ E (J) i int elec vib rot μ + T E rot Erot E C vib μ ∆E x v μ 1/νs O x Eelec Are some molecules Time YES, e.g., H , Cl , CO “Microwave inactive”? 2 2 2 3 1. Light-matter interaction Elements of spectra: Line position Line strength Rotation: Microwave Region (∆J) Line shapes Vibration: Infrared Region (∆v, J) Internal Energy : μ Eint = Eelec(n)+Evib ()+ Erot(J) t= v Erot δ+ μ s C x Evib ∆E μx O δ- Heteronuclear case is IR-active Eelec Are some vibrations “Infra-red inactive”? Yes, e.g., symmetric stretch of CO2 4 1. Light-matter interaction Summary Eint = Eelec(n)+Evib ()+ Erot(J) ∆Erot < ∆Evib < ∆Eelec Energy levels are discrete Optically allowed transitions may occur only in certain cases E rot Absorption/emission spectra are discrete Evib ∆E Current interest Rotation Rigid Rotor Non-rigid Rotor Simple Harmonic Anharmonic Eelec Vibration Oscillator Oscillator 5 2. -

The Rigid Rotor in Classical and Quantum Mechanics Paul E.S
The rigid rotor in classical and quantum mechanics paul e.s. wormer Institute of Theoretical Chemistry, University of Nijmegen, Toernooiveld, 6525 ED Nijmegen, The Netherlands Contents 1 Introduction 1 2 The mathematics of rotations in R3 2 3 The algebra of real antisymmetric matrices 8 4 The kinematics of a rigid body 11 5 Kinetic energy of a rigid rotor 14 6 The Euler equations 20 7 Quantization 21 8 Rigid rotor functions 23 9 The quantized energy levels of rigid rotors 29 10 Angular momenta and Lie derivatives 34 10.1 Infinitesimal rotations of functions f(r) ............ 35 10.2 Infinitesimal rotations of functions of Euler angles . ..... 37 1 Introduction The following text contains notes on the classical and quantum mechanical rigid rotor. The classical part is based on the books of H. Goldstein Classical Mechanics [Addison-Wesley, Reading, MA, 1980, 2nd Ed.] and V. I. Arnold, Mathematical Methods of Classical Mechanics, [Springer-Verlag, New York, 1989, 2nd Ed.]. In the following pages Goldstein’s exposition is condensed, whereas Arnold’s terse mathematical treatment is expanded. The quantum mechanical part is in the spirit of L. C. Biedenharn and J. D. Louck Angular Momentum in Quantum Physics: Theory and Application. [Addison-Wesley, Reading, MA 1981]. 1 2 The mathematics of rotations in R3 Consider a real 3 3 matrix R with columns r , r , r , i.e., R =(r , r , r ). × 1 2 3 1 2 3 The matrix R is orthogonal if r r = δ , i, j =1, 2, 3. i · j ij The matrix R is a proper rotation matrix, if it is orthogonal and if r1, r2 and r3 form a right-handed set, i.e., 3 r r = ε r . -

6 Basics of Optical Spectroscopy
Chapter 6, page 1 6 Basics of Optical Spectroscopy It is possible, with optical methods, to examine the rotational spectra of small molecules, all the Raman rotational spectra, the vibration spectra including the Raman spectra, and the electron spectra of the bonding electrons. Some of the quantum mechanical foundations of optical spectroscopy were already covered in chapter 3, and the optical methods will be the topic of chapter 7. This chapter is concerned with the relation between the structure of a substance to its rotational, and vibration spectra, and electron spectra of the bonding electrons. A few exceptions are made at the beginning of the chapter. Since the rotational spectrum of large molecules are examined using frequency variable microwave technology, this technology will be briefly explained. 6.1 Rotational Spectroscopy 6.1.1 Microwave Measurement Method Absorption measurements of the rotational transitions necessitates the existence of a permanent dipole moment. Molecules that have no permanent dipole moment, but rather an anisotropic polarizability perpendicular to the axis of rotation, can be measured with Raman scattering. Although rotational spectra of small molecules, e.g. HI, can be examined by optical methods in the distant infrared, the frequency of the rotational transitions is shifted to the range of HF spectroscopy in molecules with large moments of inertia. For this reason, rotational spectroscopy is often called microwave spectroscopy. For the production of microwaves, we could use electron time-of-flight tubes. The reflex klystron is only tunable over a small frequency range. The carcinotron (reverse wave tubes) is tunable over a larger range by variation of the accelerating voltage of the electron beam.