Example. Calculating the Rotational Energy Levels of a Molecule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

21. Orthonormal Bases
21. Orthonormal Bases The canonical/standard basis 011 001 001 B C B C B C B0C B1C B0C e1 = B.C ; e2 = B.C ; : : : ; en = B.C B.C B.C B.C @.A @.A @.A 0 0 1 has many useful properties. • Each of the standard basis vectors has unit length: q p T jjeijj = ei ei = ei ei = 1: • The standard basis vectors are orthogonal (in other words, at right angles or perpendicular). T ei ej = ei ej = 0 when i 6= j This is summarized by ( 1 i = j eT e = δ = ; i j ij 0 i 6= j where δij is the Kronecker delta. Notice that the Kronecker delta gives the entries of the identity matrix. Given column vectors v and w, we have seen that the dot product v w is the same as the matrix multiplication vT w. This is the inner product on n T R . We can also form the outer product vw , which gives a square matrix. 1 The outer product on the standard basis vectors is interesting. Set T Π1 = e1e1 011 B C B0C = B.C 1 0 ::: 0 B.C @.A 0 01 0 ::: 01 B C B0 0 ::: 0C = B. .C B. .C @. .A 0 0 ::: 0 . T Πn = enen 001 B C B0C = B.C 0 0 ::: 1 B.C @.A 1 00 0 ::: 01 B C B0 0 ::: 0C = B. .C B. .C @. .A 0 0 ::: 1 In short, Πi is the diagonal square matrix with a 1 in the ith diagonal position and zeros everywhere else. -

Multivector Differentiation and Linear Algebra 0.5Cm 17Th Santaló
Multivector differentiation and Linear Algebra 17th Santalo´ Summer School 2016, Santander Joan Lasenby Signal Processing Group, Engineering Department, Cambridge, UK and Trinity College Cambridge [email protected], www-sigproc.eng.cam.ac.uk/ s jl 23 August 2016 1 / 78 Examples of differentiation wrt multivectors. Linear Algebra: matrices and tensors as linear functions mapping between elements of the algebra. Functional Differentiation: very briefly... Summary Overview The Multivector Derivative. 2 / 78 Linear Algebra: matrices and tensors as linear functions mapping between elements of the algebra. Functional Differentiation: very briefly... Summary Overview The Multivector Derivative. Examples of differentiation wrt multivectors. 3 / 78 Functional Differentiation: very briefly... Summary Overview The Multivector Derivative. Examples of differentiation wrt multivectors. Linear Algebra: matrices and tensors as linear functions mapping between elements of the algebra. 4 / 78 Summary Overview The Multivector Derivative. Examples of differentiation wrt multivectors. Linear Algebra: matrices and tensors as linear functions mapping between elements of the algebra. Functional Differentiation: very briefly... 5 / 78 Overview The Multivector Derivative. Examples of differentiation wrt multivectors. Linear Algebra: matrices and tensors as linear functions mapping between elements of the algebra. Functional Differentiation: very briefly... Summary 6 / 78 We now want to generalise this idea to enable us to find the derivative of F(X), in the A ‘direction’ – where X is a general mixed grade multivector (so F(X) is a general multivector valued function of X). Let us use ∗ to denote taking the scalar part, ie P ∗ Q ≡ hPQi. Then, provided A has same grades as X, it makes sense to define: F(X + tA) − F(X) A ∗ ¶XF(X) = lim t!0 t The Multivector Derivative Recall our definition of the directional derivative in the a direction F(x + ea) − F(x) a·r F(x) = lim e!0 e 7 / 78 Let us use ∗ to denote taking the scalar part, ie P ∗ Q ≡ hPQi. -

Particle-On-A-Ring” Suppose a Diatomic Molecule Rotates in Such a Way That the Vibration of the Bond Is Unaffected by the Rotation
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015 Lecture 17 2/25/15 Recommended Reading Atkins & DePaula 9.5 A. Background in Rotational Mechanics Two masses m1 and m2 connected by a weightless rigid “bond” of length r rotates with angular velocity rv where v is the linear velocity. As with vibrations we can render this rotation motion in simpler form by fixing one end of the bond to the origin terminating the other end with reduced mass , and allowing the reduced mass to rotate freely For two masses m1 and m2 separated by r the center of mass (CoM) is defined by the condition mr11 mr 2 2 (17.1) where m2,1 rr1,2 mm12 (17.2) Figure 17.1: Location of center of mass (CoM0 for two masses separated by a distance r. The moment of inertia I plays the role in the rotational energy that is played by mass in translational energy. The moment of inertia is 22 I mr11 mr 2 2 (17.3) Putting 17.3 into 17.4 we obtain I r 2 (17.4) mm where 12 . mm12 We can express the rotational kinetic energy K in terms of the angular momentum. The angular momentum is defined in terms of the cross product between the position vector r and the linear momentum vector p: Lrp (17.5) prprvrsin where the angle between p and r is ninety degrees for circular motion. As a cross product, the angular momentum vector L is perpendicular to the plane defined by the r and p vectors as shown in Figure 17.2 We can use equation 17.6 to obtain an expression for the kinetic energy in terms of the angular momentum L: Figure 17.2: The angular momentum L is a cross product of the position r vector and the linear momentum p=mv vector. -

Rotational Spectroscopy
Applied Spectroscopy Rotational Spectroscopy Recommended Reading: 1. Banwell and McCash: Chapter 2 2. Atkins: Chapter 16, sections 4 - 8 Aims In this section you will be introduced to 1) Rotational Energy Levels (term values) for diatomic molecules and linear polyatomic molecules 2) The rigid rotor approximation 3) The effects of centrifugal distortion on the energy levels 4) The Principle Moments of Inertia of a molecule. 5) Definitions of symmetric , spherical and asymmetric top molecules. 6) Experimental methods for measuring the pure rotational spectrum of a molecule Microwave Spectroscopy - Rotation of Molecules Microwave Spectroscopy is concerned with transitions between rotational energy levels in molecules. Definition d Electric Dipole: p = q.d +q -q p H Most heteronuclear molecules possess Cl a permanent dipole moment -q +q e.g HCl, NO, CO, H2O... p Molecules can interact with electromagnetic radiation, absorbing or emitting a photon of frequency ω, if they possess an electric dipole moment p, oscillating at the same frequency Gross Selection Rule: A molecule has a rotational spectrum only if it has a permanent dipole moment. Rotating molecule _ _ + + t _ + _ + dipole momentp dipole Homonuclear molecules (e.g. O2, H2, Cl2, Br2…. do not have a permanent dipole moment and therefore do not have a microwave spectrum! General features of rotating systems m Linear velocity v angular velocity v = distance ω = radians O r time time v = ω × r Moment of Inertia I = mr2. A molecule can have three different moments of inertia IA, IB and IC about orthogonal axes a, b and c. 2 I = ∑miri i R Note how ri is defined, it is the perpendicular distance from axis of rotation ri Rigid Diatomic Rotors ro IB = Ic, and IA = 0. -

28. Exterior Powers
28. Exterior powers 28.1 Desiderata 28.2 Definitions, uniqueness, existence 28.3 Some elementary facts 28.4 Exterior powers Vif of maps 28.5 Exterior powers of free modules 28.6 Determinants revisited 28.7 Minors of matrices 28.8 Uniqueness in the structure theorem 28.9 Cartan's lemma 28.10 Cayley-Hamilton Theorem 28.11 Worked examples While many of the arguments here have analogues for tensor products, it is worthwhile to repeat these arguments with the relevant variations, both for practice, and to be sensitive to the differences. 1. Desiderata Again, we review missing items in our development of linear algebra. We are missing a development of determinants of matrices whose entries may be in commutative rings, rather than fields. We would like an intrinsic definition of determinants of endomorphisms, rather than one that depends upon a choice of coordinates, even if we eventually prove that the determinant is independent of the coordinates. We anticipate that Artin's axiomatization of determinants of matrices should be mirrored in much of what we do here. We want a direct and natural proof of the Cayley-Hamilton theorem. Linear algebra over fields is insufficient, since the introduction of the indeterminate x in the definition of the characteristic polynomial takes us outside the class of vector spaces over fields. We want to give a conceptual proof for the uniqueness part of the structure theorem for finitely-generated modules over principal ideal domains. Multi-linear algebra over fields is surely insufficient for this. 417 418 Exterior powers 2. Definitions, uniqueness, existence Let R be a commutative ring with 1. -

Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum
Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum We can now extend the Rigid Rotor problem to a rotation in 3D, corre- sponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordi- nates given as ∂2 ∂2 ∂2 ∂2 2 ∂ 1 1 ∂2 1 ∂ ∂ ∇2 = + + = + + + sin θ ∂x2 ∂y2 ∂z2 ∂r2 r ∂r r2 sin2 θ ∂φ2 sin θ ∂θ ∂θ For constant radius the first two terms are zero and we have 2 2 1 ∂2 1 ∂ ∂ Hˆ (θ, φ) = − ~ ∇2 = − ~ + sin θ 2m 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ We also note that Lˆ2 Hˆ = 2I where the operator for squared angular momentum is given by 1 ∂2 1 ∂ ∂ Lˆ2 = − 2 + sin θ ~ sin2 θ ∂φ2 sin θ ∂θ ∂θ The Schr¨odingerequation is given by 2 1 ∂2ψ(θ, φ) 1 ∂ ∂ψ(θ, φ) − ~ + sin θ = Eψ(θ, φ) 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ The wavefunctions are quantized in 2 directions corresponding to θ and φ. It is possible to derive the solutions, but we will not do it here. The solutions are denoted by Yl,ml (θ, φ) and are called spherical harmonics. The quantum 1 numbers take values l = 0, 1, 2, 3, .... and ml = 0, ±1, ±2, ... ± l. The energy depends only on l and is given by 2 E = l(l + 1) ~ 2I The first few spherical harmonics are given by r 1 Y = 0,0 4π r 3 Y = cos(θ) 1,0 4π r 3 Y = sin(θ)e±iφ 1,±1 8π r 5 Y = (3 cos2 θ − 1) 2,0 16π r 15 Y = cos θ sin θe±iφ 2,±1 8π r 15 Y = sin2 θe±2iφ 2,±2 32π These spherical harmonics are related to atomic orbitals in the H-atom. -

A 3D Model of the Rigid Rotor Supported by Journal Bearings)
A 3D MODEL OF THE RIGID ROTOR SUPPORTED BY JOURNAL BEARINGS) Jiří TŮMA1, Radim KLEČKA2, Jaromír ŠKUTA3 and Jiří ŠIMEK4 1 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 2 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 3 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 4 Techlab s.r.o., Sokolovská 207, 190 00 Praha 9, [email protected] Keywords: up to 7 keywords (10 pt) 1. Introduction It is known that the journal bearing with an oil film becomes instable if the rotor rotation speed crosses a certain value, which is called the Bently-Muszynska threshold [1]. To prevent the rotor instability, the active control can be employed. The arrangement of proximity probes and piezoactuators in a rotor system is shown in figure 1. It is assumed that the bushings (carrier ring), inserted into pedestals with clearance, is a movable part in two perpendicular directions while rotor is rotating. Im(r) Bushing Proximity probes Re(r) Ω Re(u) Journal Piezoactuators Im(r) Fig. 1. Journal coordinates and arrangement The research work supported by the GAČR (project no. 101/07/1345) is aimed at the design of the journal bearing active control based on the carrier ring position manipulation by the piezoactuators according to the proximity probe signals, which are a part of the closed loop including a controller. The effect of the feedback on the rotor stability is analyzed by [5]. The test stand of the TECHLAB design [1] is shown in figure 2. -

Math 395. Tensor Products and Bases Let V and V Be Finite-Dimensional
Math 395. Tensor products and bases Let V and V 0 be finite-dimensional vector spaces over a field F . Recall that a tensor product of V and V 0 is a pait (T, t) consisting of a vector space T over F and a bilinear pairing t : V × V 0 → T with the following universal property: for any bilinear pairing B : V × V 0 → W to any vector space W over F , there exists a unique linear map L : T → W such that B = L ◦ t. Roughly speaking, t “uniquely linearizes” all bilinear pairings of V and V 0 into arbitrary F -vector spaces. In class it was proved that if (T, t) and (T 0, t0) are two tensor products of V and V 0, then there exists a unique linear isomorphism T ' T 0 carrying t and t0 (and vice-versa). In this sense, the tensor product of V and V 0 (equipped with its “universal” bilinear pairing from V × V 0!) is unique up to unique isomorphism, and so we may speak of “the” tensor product of V and V 0. You must never forget to think about the data of t when you contemplate the tensor product of V and V 0: it is the pair (T, t) and not merely the underlying vector space T that is the focus of interest. In this handout, we review a method of construction of tensor products (there is another method that involved no choices, but is horribly “big”-looking and is needed when considering modules over commutative rings) and we work out some examples related to the construction. -

Concept of a Dyad and Dyadic: Consider Two Vectors a and B Dyad: It Consists of a Pair of Vectors a B for Two Vectors a a N D B
1/11/2010 CHAPTER 1 Introductory Concepts • Elements of Vector Analysis • Newton’s Laws • Units • The basis of Newtonian Mechanics • D’Alembert’s Principle 1 Science of Mechanics: It is concerned with the motion of material bodies. • Bodies have different scales: Microscropic, macroscopic and astronomic scales. In mechanics - mostly macroscopic bodies are considered. • Speed of motion - serves as another important variable - small and high (approaching speed of light). 2 1 1/11/2010 • In Newtonian mechanics - study motion of bodies much bigger than particles at atomic scale, and moving at relative motions (speeds) much smaller than the speed of light. • Two general approaches: – Vectorial dynamics: uses Newton’s laws to write the equations of motion of a system, motion is described in physical coordinates and their derivatives; – Analytical dynamics: uses energy like quantities to define the equations of motion, uses the generalized coordinates to describe motion. 3 1.1 Vector Analysis: • Scalars, vectors, tensors: – Scalar: It is a quantity expressible by a single real number. Examples include: mass, time, temperature, energy, etc. – Vector: It is a quantity which needs both direction and magnitude for complete specification. – Actually (mathematically), it must also have certain transformation properties. 4 2 1/11/2010 These properties are: vector magnitude remains unchanged under rotation of axes. ex: force, moment of a force, velocity, acceleration, etc. – geometrically, vectors are shown or depicted as directed line segments of proper magnitude and direction. 5 e (unit vector) A A = A e – if we use a coordinate system, we define a basis set (iˆ , ˆj , k ˆ ): we can write A = Axi + Ay j + Azk Z or, we can also use the A three components and Y define X T {A}={Ax,Ay,Az} 6 3 1/11/2010 – The three components Ax , Ay , Az can be used as 3-dimensional vector elements to specify the vector. -

Review a Basis of a Vector Space 1
Review • Vectors v1 , , v p are linearly dependent if x1 v1 + x2 v2 + + x pv p = 0, and not all the coefficients are zero. • The columns of A are linearly independent each column of A contains a pivot. 1 1 − 1 • Are the vectors 1 , 2 , 1 independent? 1 3 3 1 1 − 1 1 1 − 1 1 1 − 1 1 2 1 0 1 2 0 1 2 1 3 3 0 2 4 0 0 0 So: no, they are dependent! (Coeff’s x3 = 1 , x2 = − 2, x1 = 3) • Any set of 11 vectors in R10 is linearly dependent. A basis of a vector space Definition 1. A set of vectors { v1 , , v p } in V is a basis of V if • V = span{ v1 , , v p} , and • the vectors v1 , , v p are linearly independent. In other words, { v1 , , vp } in V is a basis of V if and only if every vector w in V can be uniquely expressed as w = c1 v1 + + cpvp. 1 0 0 Example 2. Let e = 0 , e = 1 , e = 0 . 1 2 3 0 0 1 3 Show that { e 1 , e 2 , e 3} is a basis of R . It is called the standard basis. Solution. 3 • Clearly, span{ e 1 , e 2 , e 3} = R . • { e 1 , e 2 , e 3} are independent, because 1 0 0 0 1 0 0 0 1 has a pivot in each column. Definition 3. V is said to have dimension p if it has a basis consisting of p vectors. Armin Straub 1 [email protected] This definition makes sense because if V has a basis of p vectors, then every basis of V has p vectors. -
Rotational Spectroscopy
Rotational spectroscopy - Involve transitions between rotational states of the molecules (gaseous state!) - Energy difference between rotational levels of molecules has the same order of magnitude with microwave energy - Rotational spectroscopy is called pure rotational spectroscopy, to distinguish it from roto-vibrational spectroscopy (the molecule changes its state of vibration and rotation simultaneously) and vibronic spectroscopy (the molecule changes its electronic state and vibrational state simultaneously) Molecules do not rotate around an arbitrary axis! Generally, the rotation is around the mass center of the molecule. The rotational axis must allow the conservation of M R α pα const kinetic angular momentum. α Rotational spectroscopy Rotation of diatomic molecule - Classical description Diatomic molecule = a system formed by 2 different masses linked together with a rigid connector (rigid rotor = the bond length is assumed to be fixed!). The system rotation around the mass center is equivalent with the rotation of a particle with the mass μ (reduced mass) around the center of mass. 2 2 2 2 m1m2 2 The moment of inertia: I miri m1r1 m2r2 R R i m1 m2 Moment of inertia (I) is the rotational equivalent of mass (m). Angular velocity () is the equivalent of linear velocity (v). Er → rotational kinetic energy L = I → angular momentum mv 2 p2 Iω2 L2 E E c 2 2m r 2 2I Quantum rotation: The diatomic rigid rotor The rigid rotor represents the quantum mechanical “particle on a sphere” problem: Rotational energy is purely -
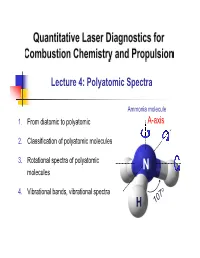
Lecture 4: Polyatomic Spectra
Lecture 4: Polyatomic Spectra Ammonia molecule 1. From diatomic to polyatomic A-axis 2. Classification of polyatomic molecules 3. Rotational spectra of polyatomic N molecules 4. Vibrational bands, vibrational spectra H 1. From diatomic to polyatomic Rotation – Diatomics Recall: For diatomic molecules Energy: FJ ,cm1 BJJ 1 DJ 2 J 12 R.R. Centrifugal distortion constant h Rotational constant: B,cm1 8 2 Ic Selection Rule: J ' J"1 J 1 3 Line position: J "1J " 2BJ"1 4D J"1 Notes: 2 1. D is small, i.e., D / B 4B / vib 1 2 2 D B 1.7 6 2. E.g., for NO, 4 4 310 B NO e 1900 → Even @ J=60, D / B J 2 ~ 0.01 What about polyatomics (≥3 atoms)? 2 1. From diatomic to polyatomic 3D-body rotation B . Convention: A A-axis is the “unique” or “figure” axis, along which lies the molecule’s C defining symmetry . 3 principal axes (orthogonal): A, B, C . 3 principal moments of inertia: IA, IB, IC . Molecules are classified in terms of the relative values of IA, IB, IC 3 2. Classification of polyatomic molecules Types of molecules Linear Symmetric Asymmetric Type Spherical Tops Molecules Tops Rotors Relative IB=IC≠IA magnitudes IB=IC; IA≈0* IA=IB=IC IA≠IB≠IC IA≠0 of IA,B,C NH CO2 3 H2O CH4 C2H2 CH F Examples 3 Acetylene NO2 OCS BCl3 Carbon Boron oxysulfide trichloride Relatively simple No dipole moment Largest category Not microwave active Most complex *Actually finite, but quantized momentum means it is in lowest state of rotation 4 2.