Rotational Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vibrational-Rotational Spectroscopy ( )
Vibrational-Rotational Spectroscopy Vibrational-Rotational Spectrum of Heteronuclear Diatomic Absorption of mid-infrared light (~300-4000 cm-1): • Molecules can change vibrational and rotational states • Typically at room temperature, only ground vibrational state populated but several rotational levels may be populated. • Treating as harmonic oscillator and rigid rotor: subject to selection rules ∆v = ±1 and ∆J = ±1 EEEfield=∆ vib +∆ rot =ω =−EEfi = EvJEvJ()′,, ′ −( ′′ ′′ ) ω 11 vvvBJJvvBJJ==00()′′′′′′′′′ +++−++()11() () + 2πc 22 At room temperature, typically v=0′′ and ∆v = +1: ′ ′ ′′ ′′ vv=+0 BJJ() +−11 JJ( +) Now, since higher lying rotational levels can be populated, we can have: ∆=+J 1 JJ′′′=+1 vv= ++21 BJ( ′′ ) R − branch 0 P− branch ∆=−J 1 JJ′′′=−1 vv=−0 2 BJ′′ J’=4 J’=3 J’=1 v’=1 J’=0 P branch Q branch J’’=3 12B J’’=2 6B J’’=1 2B v’’=0 J’’=0 EJ” =0 2B 2B 4B 2B 2B 2B -6B -4B -2B +2B +4B +8B ν ν 0 By measuring absorption splittings, we can get B . From that, the bond length! In polyatomics, we can also have a Q branch, where ∆J0= and all transitions lie at ν=ν0 . This transition is allowed for perpendicular bands: ∂µ ∂q ⊥ to molecular symmetry axis. Intensity of Vibrational-Rotational Transitions There is generally no thermal population in upper (final) state (v’,J’) so intensity should scale as population of lower J state (J”). ∆=NNvJNvJ(,′ ′′′′′′′ ) − (, ) ≈ NJ ( ) NJ()′′∝− gJ ()exp( ′′ EJ′′ / kT ) =+()21exp(J′′ −hcBJ ′′() J ′′ + 1/) kT 5.33 Lecture Notes: Vibrational-Rotational Spectroscopy Page 2 Rotational Populations at Room Temperature for B = 5 cm-1 gJ'' thermal population NJ'' 0 5 10 15 20 Rotational Quantum Number J'' So, the vibrational-rotational spectrum should look like equally spaced lines about ν 0 with sidebands peaked at J’’>0. -
Optical Spectroscopy - Processes Monitored UV/ Fluorescence/ IR/ Raman/ Circular Dichroism
Time out—states and transitions Spectroscopy—transitions between energy states of a molecule excited by absorption or emission of a photon hν = ∆E = Ei -Ef Energy levels due to interactions between parts of molecule (atoms, electrons and nucleii) as described by quantum mechanics, and are characteristic of components involved, i.e. electron distributions (orbitals), bond strengths and types plus molecular geometries and atomic masses involved Spectroscopic Regions Typical wavelength Approximate energy Spectroscopic region Techniques and Applications (cm) (kcal mole-1) -11 8 10 3 x 10 γ-ray MÖssbauer 10-8 3 x 105 X-ray x-ray diffraction, scattering 10-5 3 x 102 Vacuum UV Electronic Spectra 3 x 10-5 102 Near UV Electronic Spectra 6 x 10-5 5 x 103 Visible Electronic Spectra 10-3 3 x 100 IR Vibrational Spectra 10-2 3 x 10-1 Far IR Vibrational Spectra 10-1 3 x 10-2 Microwave Rotational Spectra 100 3 x 10-3 Microwave Electron paramagnetic resonance 10 3 x 10-4 Radio frequency Nuclear magnetic resonance Adapted from Table 7-1; Biophysical Chemistry, Part II by Cantor and Schimmel Spectroscopic Process • Molecules contain distribution of charges (electrons and nuclei, charges from protons) and spins which is dynamically changed when molecule is exposed to light •In a spectroscopic experiment, light is used to probe a sample. What we seek to understand is: – the RATE at which the molecule responds to this perturbation (this is the response or spectral intensity) – why only certain wavelengths cause changes (this is the spectrum, the wavelength dependence of the response) – the process by which the molecule alters the radiation that emerges from the sample (absorption, scattering, fluorescence, photochemistry, etc.) so we can detect it These tell us about molecular identity, structure, mechanisms and analytical concentrations Magnetic Resonance—different course • Long wavelength radiowaves are of low energy that is sufficient to ‘flip’ the spin of nuclei in a magnetic field (NMR). -

Rotational Spectroscopy and Interstellar Molecules
Volume -5, Issue-2, April 2015 » Plescia, J. B., and Cintala M. J. (2012), Impact melt Rotational Spectroscopy and Interstellar in small lunar highland craters, J. Geophys. Res., 117, Molecules E00H12,doi:10.1029/2011JE003941 » Plescia J.B. and Spudis P.D. (2014) Impact melt flows at The fact that life exists on Earth is no secret. However, Lowell crater, Planetary and Space Science, 103, 219- understanding the origin of life, its evolution, and the fu- 227 ture of life on Earth remain interesting issues to be ad- » Shkuratov, Y.,Kaydash, V.,Videen, G. (2012)The lunar dressed. That the regions between stars contain by far the crater Giordano Bruno as seen with optical roughness largest reservoir of chemically-bonded matter in nature imagery, Icarus, 218(1), 525-533 obviously demonstrates the importance of chemistry in » Smrekar S. and Pieters C.M. (1985) Near-infrared spec- the interstellar space. The unique detection of over 200 troscopy of probable impact melt from three large lunar different interstellar molecules largely via their rotational highland craters, Icarus, 63, 442-452 spectra has laid to rest the popular perception that the » Srivastava, N., Kumar, D., Gupta, R. P., 2013. Young vastness of space is an empty vacuum dotted with stars, viscous flows in the Lowell crater of Orientale basin, planets, black holes, and other celestial formations. As- Moon: Impact melts or volcanic eruptions? Planetary trochemistry comprises observations, theory and experi- and Space Science, 87, 37-45. ments aimed at understanding the formation of molecules » Stopar J. D., Hawke B. R., Robinson M. S., DeneviB.W., and matter in the Universe i.e. -

Particle-On-A-Ring” Suppose a Diatomic Molecule Rotates in Such a Way That the Vibration of the Bond Is Unaffected by the Rotation
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015 Lecture 17 2/25/15 Recommended Reading Atkins & DePaula 9.5 A. Background in Rotational Mechanics Two masses m1 and m2 connected by a weightless rigid “bond” of length r rotates with angular velocity rv where v is the linear velocity. As with vibrations we can render this rotation motion in simpler form by fixing one end of the bond to the origin terminating the other end with reduced mass , and allowing the reduced mass to rotate freely For two masses m1 and m2 separated by r the center of mass (CoM) is defined by the condition mr11 mr 2 2 (17.1) where m2,1 rr1,2 mm12 (17.2) Figure 17.1: Location of center of mass (CoM0 for two masses separated by a distance r. The moment of inertia I plays the role in the rotational energy that is played by mass in translational energy. The moment of inertia is 22 I mr11 mr 2 2 (17.3) Putting 17.3 into 17.4 we obtain I r 2 (17.4) mm where 12 . mm12 We can express the rotational kinetic energy K in terms of the angular momentum. The angular momentum is defined in terms of the cross product between the position vector r and the linear momentum vector p: Lrp (17.5) prprvrsin where the angle between p and r is ninety degrees for circular motion. As a cross product, the angular momentum vector L is perpendicular to the plane defined by the r and p vectors as shown in Figure 17.2 We can use equation 17.6 to obtain an expression for the kinetic energy in terms of the angular momentum L: Figure 17.2: The angular momentum L is a cross product of the position r vector and the linear momentum p=mv vector. -

Photoionization Spectroscopy O
Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, A. Flütsch, C. Romanzin, J.-C. Guillemin, Séverine Boyé-Péronne, B. Gans To cite this version: N. Lamarre, C. Falvo, C. Alcaraz, B. Cunha de Miranda, S. Douin, et al.. Photoionization spectroscopy of CH3C3N in the vacuum-ultraviolet range. Journal of Molecular Spectroscopy, Elsevier, 2015, 315, pp.206-216. 10.1016/j.jms.2015.03.005. hal-01138635 HAL Id: hal-01138635 https://hal-univ-rennes1.archives-ouvertes.fr/hal-01138635 Submitted on 4 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Photoionization spectroscopy of CH 3C3N in the vacuum-ultraviolet range N. Lamarre a, C. Falvo a, C. Alcaraz b,c, B. Cunha de Miranda b, S. Douin a, A. Fl utsch¨ a, C. Romanzin b, J.-C. Guillemin d, a, a, S. Boy e-P´ eronne´ ∗, B. Gans ∗ aInstitut des Sciences Mol´eculaires d’Orsay, Univ Paris-Sud; CNRS, bat 210, Univ Paris-Sud 91405 Orsay cedex (France) bLaboratoire de Chimie Physique, Univ Paris-Sud; CNRS UMR 8000, bat 350, Univ Paris-Sud 91405 Orsay cedex (France) cSynchrotron SOLEIL, L'Orme des Merisiers, St. -

Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum
Lecture 6: 3D Rigid Rotor, Spherical Harmonics, Angular Momentum We can now extend the Rigid Rotor problem to a rotation in 3D, corre- sponding to motion on the surface of a sphere of radius R. The Hamiltonian operator in this case is derived from the Laplacian in spherical polar coordi- nates given as ∂2 ∂2 ∂2 ∂2 2 ∂ 1 1 ∂2 1 ∂ ∂ ∇2 = + + = + + + sin θ ∂x2 ∂y2 ∂z2 ∂r2 r ∂r r2 sin2 θ ∂φ2 sin θ ∂θ ∂θ For constant radius the first two terms are zero and we have 2 2 1 ∂2 1 ∂ ∂ Hˆ (θ, φ) = − ~ ∇2 = − ~ + sin θ 2m 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ We also note that Lˆ2 Hˆ = 2I where the operator for squared angular momentum is given by 1 ∂2 1 ∂ ∂ Lˆ2 = − 2 + sin θ ~ sin2 θ ∂φ2 sin θ ∂θ ∂θ The Schr¨odingerequation is given by 2 1 ∂2ψ(θ, φ) 1 ∂ ∂ψ(θ, φ) − ~ + sin θ = Eψ(θ, φ) 2mR2 sin2 θ ∂φ2 sin θ ∂θ ∂θ The wavefunctions are quantized in 2 directions corresponding to θ and φ. It is possible to derive the solutions, but we will not do it here. The solutions are denoted by Yl,ml (θ, φ) and are called spherical harmonics. The quantum 1 numbers take values l = 0, 1, 2, 3, .... and ml = 0, ±1, ±2, ... ± l. The energy depends only on l and is given by 2 E = l(l + 1) ~ 2I The first few spherical harmonics are given by r 1 Y = 0,0 4π r 3 Y = cos(θ) 1,0 4π r 3 Y = sin(θ)e±iφ 1,±1 8π r 5 Y = (3 cos2 θ − 1) 2,0 16π r 15 Y = cos θ sin θe±iφ 2,±1 8π r 15 Y = sin2 θe±2iφ 2,±2 32π These spherical harmonics are related to atomic orbitals in the H-atom. -

A 3D Model of the Rigid Rotor Supported by Journal Bearings)
A 3D MODEL OF THE RIGID ROTOR SUPPORTED BY JOURNAL BEARINGS) Jiří TŮMA1, Radim KLEČKA2, Jaromír ŠKUTA3 and Jiří ŠIMEK4 1 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 2 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 3 VSB – Technical University of Ostrava, Ostrava, Czech Republic, [email protected] 4 Techlab s.r.o., Sokolovská 207, 190 00 Praha 9, [email protected] Keywords: up to 7 keywords (10 pt) 1. Introduction It is known that the journal bearing with an oil film becomes instable if the rotor rotation speed crosses a certain value, which is called the Bently-Muszynska threshold [1]. To prevent the rotor instability, the active control can be employed. The arrangement of proximity probes and piezoactuators in a rotor system is shown in figure 1. It is assumed that the bushings (carrier ring), inserted into pedestals with clearance, is a movable part in two perpendicular directions while rotor is rotating. Im(r) Bushing Proximity probes Re(r) Ω Re(u) Journal Piezoactuators Im(r) Fig. 1. Journal coordinates and arrangement The research work supported by the GAČR (project no. 101/07/1345) is aimed at the design of the journal bearing active control based on the carrier ring position manipulation by the piezoactuators according to the proximity probe signals, which are a part of the closed loop including a controller. The effect of the feedback on the rotor stability is analyzed by [5]. The test stand of the TECHLAB design [1] is shown in figure 2. -

Hydrogen Conversion in Nanocages
Review Hydrogen Conversion in Nanocages Ernest Ilisca Laboratoire Matériaux et Phénomènes Quantiques, Université de Paris, CNRS, F-75013 Paris, France; [email protected] Abstract: Hydrogen molecules exist in the form of two distinct isomers that can be interconverted by physical catalysis. These ortho and para forms have different thermodynamical properties. Over the last century, the catalysts developed to convert hydrogen from one form to another, in laboratories and industries, were magnetic and the interpretations relied on magnetic dipolar interactions. The variety concentration of a sample and the conversion rates induced by a catalytic action were mostly measured by thermal methods related to the diffusion of the o-p reaction heat. At the turning of the new century, the nature of the studied catalysts and the type of measures and motivations completely changed. Catalysts investigated now are non-magnetic and new spectroscopic measurements have been developed. After a fast survey of the past studies, the review details the spectroscopic methods, emphasizing their originalities, performances and refinements: how Infra-Red measurements charac- terize the catalytic sites and follow the conversion in real-time, Ultra-Violet irradiations explore the electronic nature of the reaction and hyper-frequencies driving the nuclear spins. The new catalysts, metallic or insulating, are detailed to display the operating electronic structure. New electromagnetic mechanisms, involving energy and momenta transfers, are discovered providing a classification frame for the newly observed reactions. Keywords: molecular spectroscopy; nanocages; electronic excitations; nuclear magnetism Citation: Ilisca, E. Hydrogen Conversion in Nanocages. Hydrogen The intertwining of quantum, spectroscopic and thermodynamical properties of the 2021, 2, 160–206. -
Rotational Spectroscopy
Rotational spectroscopy - Involve transitions between rotational states of the molecules (gaseous state!) - Energy difference between rotational levels of molecules has the same order of magnitude with microwave energy - Rotational spectroscopy is called pure rotational spectroscopy, to distinguish it from roto-vibrational spectroscopy (the molecule changes its state of vibration and rotation simultaneously) and vibronic spectroscopy (the molecule changes its electronic state and vibrational state simultaneously) Molecules do not rotate around an arbitrary axis! Generally, the rotation is around the mass center of the molecule. The rotational axis must allow the conservation of M R α pα const kinetic angular momentum. α Rotational spectroscopy Rotation of diatomic molecule - Classical description Diatomic molecule = a system formed by 2 different masses linked together with a rigid connector (rigid rotor = the bond length is assumed to be fixed!). The system rotation around the mass center is equivalent with the rotation of a particle with the mass μ (reduced mass) around the center of mass. 2 2 2 2 m1m2 2 The moment of inertia: I miri m1r1 m2r2 R R i m1 m2 Moment of inertia (I) is the rotational equivalent of mass (m). Angular velocity () is the equivalent of linear velocity (v). Er → rotational kinetic energy L = I → angular momentum mv 2 p2 Iω2 L2 E E c 2 2m r 2 2I Quantum rotation: The diatomic rigid rotor The rigid rotor represents the quantum mechanical “particle on a sphere” problem: Rotational energy is purely -
Lecture 4: Polyatomic Spectra
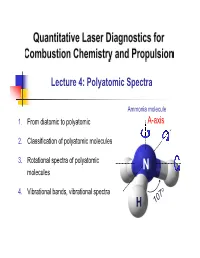
Lecture 4: Polyatomic Spectra Ammonia molecule 1. From diatomic to polyatomic A-axis 2. Classification of polyatomic molecules 3. Rotational spectra of polyatomic N molecules 4. Vibrational bands, vibrational spectra H 1. From diatomic to polyatomic Rotation – Diatomics Recall: For diatomic molecules Energy: FJ ,cm1 BJJ 1 DJ 2 J 12 R.R. Centrifugal distortion constant h Rotational constant: B,cm1 8 2 Ic Selection Rule: J ' J"1 J 1 3 Line position: J "1J " 2BJ"1 4D J"1 Notes: 2 1. D is small, i.e., D / B 4B / vib 1 2 2 D B 1.7 6 2. E.g., for NO, 4 4 310 B NO e 1900 → Even @ J=60, D / B J 2 ~ 0.01 What about polyatomics (≥3 atoms)? 2 1. From diatomic to polyatomic 3D-body rotation B . Convention: A A-axis is the “unique” or “figure” axis, along which lies the molecule’s C defining symmetry . 3 principal axes (orthogonal): A, B, C . 3 principal moments of inertia: IA, IB, IC . Molecules are classified in terms of the relative values of IA, IB, IC 3 2. Classification of polyatomic molecules Types of molecules Linear Symmetric Asymmetric Type Spherical Tops Molecules Tops Rotors Relative IB=IC≠IA magnitudes IB=IC; IA≈0* IA=IB=IC IA≠IB≠IC IA≠0 of IA,B,C NH CO2 3 H2O CH4 C2H2 CH F Examples 3 Acetylene NO2 OCS BCl3 Carbon Boron oxysulfide trichloride Relatively simple No dipole moment Largest category Not microwave active Most complex *Actually finite, but quantized momentum means it is in lowest state of rotation 4 2. -

Fine-And Hyperfine-Structure Effects in Molecular Photoionization: I
Fine- and hyperfine-structure effects in molecular photoionization: I. General theory and direct photoionization Matthias Germann1 and Stefan Willitsch1, a) Department of Chemistry, University of Basel, Klingelbergstrasse 80, 4056 Basel, Switzerland (Dated: 2 October 2018) We develop a model for predicting fine- and hyperfine intensities in the direct photoionization of molecules based on the separability of electron and nuclear spin states from vibrational-electronic states. Using spherical tensor algebra, we derive highly symmetrized forms of the squared photoionization dipole matrix elements from which which we derive the salient selection and propensity rules for fine- and hyperfine resolved photoionizing transitions. Our theoretical results are validated by the analysis of the fine-structure resolved photoelectron spectrum of O2 (reported by H. Palm and F. Merkt, Phys. Rev. Lett. 81, 1385 (1998)) and are used for predicting hyperfine populations of molecular ions produced by photoionization. I. INTRODUCTION dynamics.19–21 Thus, there is a growing need for theo- retical models capable of describing fine- and hyperfine Photoionization and photoelectron spectroscopy are effects in molecular photoionization. Whereas the hy- among the eminent experimental techniques to gain infor- perfine structure in Rydberg spectra has previously been mation on the electronic structure of molecules, on their treated within the framework of multichannel quantum- 14–18 photoionization dynamics and the structure and dynam- defect theory (MQDT), we are not aware of -

INTRODUCTORY LECTURE on ROTATIONAL SPECTROSCOPY
INTRODUCTORYINTRODUCTORY LECTURELECTURE onon ROTATIONALROTATIONAL SPECTROSCOPYSPECTROSCOPY CristinaCristina PuzzariniPuzzarini Dip.Dip. ChimicaChimica ““GiacomoGiacomo CiamicianCiamician”” UniversitUniversitàà didi BolognaBologna CODECSCODECS SummerSummer SchoolSchool 20132013 THEORETICALTHEORETICAL SPECTROSCOPYSPECTROSCOPY EE Eel Evib Erot ELECTRONICELECTRONIC VIBRATIONALVIBRATIONAL ROTATIONALROTATIONAL FREQUENCYFREQUENCY REGIONREGION mm/submm waves Rotational Spectroscopy Electronics Photonics “Building”“Building” thethe ROTATIONALROTATIONAL SPECTRUMSPECTRUM intensity (a.u.) 0 20 40 60 80 100 frequency (cm-1) (1)(1) RotationalRotational energyenergy levelslevels (2)(2) SelectionSelection rules:rules: transitionstransitions allowedallowed (3)(3) IntensityIntensity (transitions)(transitions) CoordinateCoordinate SSyystemstemss Molecule-fixed coordinate system Lab-fixed coordinate system ROTATINGROTATING RIGIDRIGID BODYBODY (CLASSIC(CLASSIC VIEW)VIEW) 1 T ωTIω 2 == angolarangolar velocityvelocity II == inertiainertia tensortensor AngolarAngolar VelocitVelocityy vi r i x ω y RIGIDRIGID BODY:BODY: v ωr i i z InertiaInertia TensorTensor I xx I xy I xz n n 2 2 2 2 I xx mi ri xi mi yi zi i1 i1 I I yx I yy I yz n I xy mi xi yi i1 I zx I zy I zz ByBy cconvenonventiontion:: IIc IIb IIa INERTIAINERTIA TENSORTENSOR II Principal Principal I xx I xy I xz I x 0 0 inertiainertia I I yx I yy I yz systemsystem I 0 I y 0 I zx I zy I zz 0 0 I z AngularAngular MomentMoment RIGID BODY J e defined in the rotating