Thermoregulatory Competence and Behavioral Expression in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tissue Tregs and Maintenance of Tissue Homeostasis
fcell-09-717903 August 12, 2021 Time: 13:32 # 1 REVIEW published: 18 August 2021 doi: 10.3389/fcell.2021.717903 Tissue Tregs and Maintenance of Tissue Homeostasis Qing Shao1,2,3,4†, Jian Gu1,2,3,4†, Jinren Zhou1,2,3,4†, Qi Wang1,2,3,4, Xiangyu Li1,2,3,4, Zhenhua Deng1,2,3,4 and Ling Lu1,2,3,4* 1 Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2 Research Unit of Liver Transplantation and Transplant Immunology, Chinese Academy of Medical Sciences, Nanjing, China, 3 Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing Medical University Affiliated Cancer Hospital, Nanjing, China, 4 Jiangsu Key Laboratory of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China Regulatory T cells (Tregs) specifically expressing Forkhead box P3 (Foxp3) play roles in suppressing the immune response and maintaining immune homeostasis. Edited by: After maturation in the thymus, Tregs leave the thymus and migrate to lymphoid Ivan Dzhagalov, tissues or non-lymphoid tissues. Increasing evidence indicates that Tregs with unique National Yang-Ming University, Taiwan characteristics also have significant effects on non-lymphoid peripheral tissues. Tissue- Reviewed by: resident Tregs, also called tissue Tregs, do not recirculate in the blood or lymphatics Dipayan Rudra, ImmunoBiome Inc., South Korea and attain a unique phenotype distinct from common Tregs in circulation. This review Ying Shao, first summarizes the phenotype, function, and cytokine expression of these Tregs in Temple University, United States visceral adipose tissue, skin, muscle, and other tissues. Then, how Tregs are generated, *Correspondence: Ling Lu home, and are attracted to and remain resident in the tissue are discussed. -

On Normal Tissue Homeostasis Maintenance of Immune Tolerance
Maintenance of Immune Tolerance Depends on Normal Tissue Homeostasis Zita F. H. M. Boonman, Geertje J. D. van Mierlo, Marieke F. Fransen, Rob J. W. de Keizer, Martine J. Jager, Cornelis J. This information is current as M. Melief and René E. M. Toes of September 27, 2021. J Immunol 2005; 175:4247-4254; ; doi: 10.4049/jimmunol.175.7.4247 http://www.jimmunol.org/content/175/7/4247 Downloaded from References This article cites 48 articles, 21 of which you can access for free at: http://www.jimmunol.org/content/175/7/4247.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 27, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2005 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Maintenance of Immune Tolerance Depends on Normal Tissue Homeostasis Zita F. H. M. Boonman,* Geertje J. D. van Mierlo,† Marieke F. Fransen,† Rob J. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

Tissue Engineering: from Cell Biology to Artificial Organs
干细胞之家www.stemcell8.cn ←点击进入 Tissue Engineering Essentials for Daily Laboratory Work W. W. Minuth, R. Strehl, K. Schumacher 干细胞之家www.stemcell8.cn ←点击进入 干细胞之家www.stemcell8.cn ←点击进入 Tissue Engineering W. W. Minuth, R. Strehl, K. Schumacher 干细胞之家www.stemcell8.cn ←点击进入 Further Titles of Interest Novartis Foundation Symposium Kay C. Dee, David A. Puleo, Rena Bizios Tissue Engineering An Introduction to Tissue- of Cartilage and Bone – Biomaterial Interactions No. 249 2002 ISBN 0-471-25394-4 2003 ISBN 0-470-84481-7 Alan Doyle, J. Bryan Griffiths (Eds.) Rolf D. Schmid, Ruth Hammelehle Cell and Tissue Culture Pocket Guide to Biotechnology for Medical Research and Genetic Engineering 2000 2003 ISBN 0-471-85213-9 ISBN 3-527-30895-4 R. Ian Freshney Michael Hoppert Culture of Animal Cells: Microscopic Techniques A Manual of Basic Technique, in Biotechnology 4th Edition 2003 ISBN 3-527-30198-4 2000 ISBN 0-471-34889-9 R. Ian Freshney, Mary G. Freshney Oliver Kayser, Rainer H. Mu¨ller (Eds.) (Eds.) Pharmaceutical Biotechnology: Culture of Epithelial Cells, Drug Discovery and Clinical 2nd Edition Applications 2002 2004 ISBN 0-471-40121-8 ISBN 3-527-30554-8 干细胞之家www.stemcell8.cn ←点击进入 Tissue Engineering Essentials for Daily Laboratory Work W. W. Minuth, R. Strehl, K. Schumacher 干细胞之家www.stemcell8.cn ←点击进入 Authors This book was carefully produced. Nevertheless, editors, authors and publisher do not warrant the Dr. Will W. Minuth, PhD information contained therein to be free of errors. Raimund Strehl, PhD Readers are advised to keep in mind that state- Karl Schumacher, M.D. ments, data, illustrations, procedural details or other items may inadvertently be inaccurate. -

Psychology of Pain KENNETH D
Postgrad Med J: first published as 10.1136/pgmj.60.710.835 on 1 December 1984. Downloaded from Postgraduate Medical Journal (December 1984) 60, 835-840 Psychology of pain KENNETH D. CRAIG M.A., Ph.D. Department of Psychology, University of British Columbia, Vancouver, B.C. Canada V6T 1 Y7 Introduction Many chronic pain syndromes, as well as some reactions to acute pain, can only be understood by To the sufferer, pain is a vital reality. While fully incorporating psychological variables into explana- aware of this, the scientist and practitioner must also tory models. Exclusively sensory and predominantly recognize that efforts to understand and manage pain biophysical explanatory models, that emphasize can be only as good as the available theoretical treatment of underlying pathophysiological pro- models. Recent decades have seen concepts of pain cesses, have proved inadequate, with large numbers increasingly embrace psychological models (Merskey of patients who do not benefit from care based on this and Spear, 1967; Stembach, 1978; Melzack and Wall, model. While the majority of painful injuries heal 1983). The definition of pain adopted by the Interna- through spontaneous recovery and medical interven- tional Association for the Study of Pain (1979) tion, Bonica (1983) has estimated that one-third of describes pain as 'An unpleasant sensory and emo- the population suffers some form of recurrent or tional experience associated with actual or potential persistent pain. tissue damage, or described in terms ofsuch damage'. by copyright. -

Tissue Homeostasis
Chapter 3 Tissue homeostasis Anders Lindahl Chapter contents 3.5 Tissues where regeneration was not considered – the paradigm shift in 3.1 Introduction 74 tissue regeneration 79 3.2 Tissues with no potential of 3.6 Consequence of regeneration potential regeneration 76 for the tissue engineering concept 81 3.3 Tissues with slow regeneration time 76 3.7 Cell migration of TA cells 85 3.4 Tissues with a high capacity of 3.8 Future developments 86 regeneration 77 3.9 Summary 86 Chapter objectives: ● To know the definition of the terms ● To recognize a stem cell niche regeneration and homeostasis ● To understand that stem cell niches ● To recognize that different tissues have share common regulatory systems different regeneration capacity ● To understand that tissue regeneration ● To acknowledge that the brain and heart has consequences and offer are regenerating organs opportunities in the development of ● To understand how tissue regeneration tissue engineering products can be analyzed by labeling experiments CCH003.inddH003.indd 7733 11/29/2008/29/2008 33:18:21:18:21 PPMM 74 Chapter 3 Tissue homeostasis I ’ d give my right arm to know the secret of regeneration Oscar E Schotte, quoted in Goss (1991) . 3.1 Introduction Distal amputation The ability to regenerate larger parts of an organism Original is connected to the complexity of that organism. The limp lower developed the animal, the better the regen- eration ability. In most vertebrates, the regeneration potential is limited to the musculoskeletal system and Amputation liver. In the hydras (a 0.5 cm long fresh-water cnidar- ian) the regeneration is made through morphollaxis, a process that does not require any cell division. -

Mechanistic Ideas of Life: the Cell Theory
Mechanistic Ideas of Life: The Cell Theory Robert Hooke-1665 • Examined thin slices of cork and discovered: "Yet it was not unlike a Honey-comb in these particulars...these pores, or cells, ... consisted of a great many little Boxes.... Nor is this kind of texture peculiar to Cork onely; for upon examination with my Microscope, I have found that the pith of an Elder, or almost any other Tree, the inner pulp or pith of ... several other Vegetables ... have much such a kind of Schematisme, as I have lately shown [in] that of Cork." • Hooke called them “cellulae” (Latin word for “little rooms” ). • Cells defined by their walls Antony van Leeuwenhoek • Developed his own single-lens microscopes for use on fabrics (operated a drapery business in Delft) • First to observe details of animal structure (muscle banding) as well as single-celled organisms (bacteria, sperm) – Sent results to the new Royal Society 1 Jan Swammerdam: (1637-1680) Describes the appearance of blood under microscope: “If we begin the dissection in the upper part of the abdomen, and cautiously split the skin there, blood immediately escapes from that place. The blood, when received into a glass tube and examined with a very good microscope, is observed to consist of transparent globules (globulis), in no way differing from cow's milk, a fact that was discovered a few years ago in human blood also; for this is seen to consist of slightly reddish globules, floating in a clear fluid.” Marie François Xavier Bichat (1800): doing without microscopes Rejected the value of observing with microscopes, but nonetheless made very astute observations: • Two different sets of organs – Those under volitional control and serving locomotion – Those serving vital processes: digestion, assimilation, etc. -

An Introduction to Stem Cell Biology
An Introduction to Stem Cell Biology Michael L. Shelanski, MD,PhD Professor of Pathology and Cell Biology Columbia University Figures adapted from ISSCR. Presentations of Drs. Martin Pera (Monash University), Dr.Susan Kadereit, Children’s Hospital, Boston and Dr. Catherine Verfaillie, University of Minnesota Science 1999, 283: 534-537 PNAS 1999, 96: 14482-14486 Turning Blood into Brain: Cells Bearing Neuronal Antigens Generated in Vitro from Bone Marrow Science 2000, 290:1779-1782 From Marrow to Brain: Expression of Neuronal Phenotypes in Adult Mice Mezey, E., Chandross, K.J., Harta, G., Maki, R.A., McKercher, S.R. Science 2000, 290:1775-1779 Brazelton, T.R., Rossi, F.M., Keshet, G.I., Blau, H.M. Nature 2001, 410:701-705 Nat Med 2000, 11: 1229-1234 Stem Cell FAQs Do you need to get one from an egg? Must you sacrifice an Embryo? What is an ES cell? What about adult stem cells or cord blood stem cells Why can’t this work be done in animals? Are “cures” on the horizon? Will this lead to human cloning – human spare parts factories? Are we going to make a Frankenstein? What is a stem cell? A primitive cell which can either self renew (reproduce itself) or give rise to more specialised cell types The stem cell is the ancestor at the top of the family tree of related cell types. One blood stem cell gives rise to red cells, white cells and platelets Stem Cells Vary in their Developmental capacity A multipotent cell can give rise to several types of mature cell A pluripotent cell can give rise to all types of adult tissue cells plus extraembryonic tissue: cells which support embryonic development A totipotent cell can give rise to a new individual given appropriate maternal support The Fertilized Egg The “Ultimate” Stem Cell – the Newly Fertilized Egg (one Cell) will give rise to all the cells and tissues of the adult animal. -

The History and Scope of Tissue Engineering Joseph Vacanti and Charles A
Chapter One The History and Scope of Tissue Engineering Joseph Vacanti and Charles A. Vacanti I. Introduction V. General Scientifi c Issues II. Scientifi c Challenges VI. Social Challenges III. Cells VII. References IV. Materials I. INTRODUCTION the mid-19th century enabled the rapid evolution of many The dream is as old as humankind. Injury, disease, and surgical techniques. With patients anesthetized, innovative congenital malformation have always been part of the and courageous surgeons could save lives by examining human experience. If only damaged bodies could be and treating internal areas of the body: the thorax, the restored, life could go on for loved ones as though tragedy abdomen, the brain, and the heart. Initially the surgical had not intervened. In recorded history, this possibility fi rst techniques were primarily extirpative, for example, removal was manifested through myth and magic, as in the Greek of tumors, bypass of the bowel in the case of intestinal legend of Prometheus and eternal liver regeneration. Then obstruction, and repair of life-threatening injuries. Main- legend produced miracles, as in the creation of Eve in tenance of life without regard to the crippling effects of Genesis or the miraculous transplantation of a limb by the tissue loss or the psychosocial impact of disfi gurement, saints Cosmos and Damien. With the introduction of the however, was not an acceptable end goal. Techniques that scientifi c method came new understanding of the natural resulted in the restoration of function through structural world. The methodical unraveling of the secrets of biology replacement became integral to the advancement of human was coupled with the scientifi c understanding of disease therapy. -

A Short History of Imuregen – an Original Tissue Extract
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2019, 88(3), 115-120 ISSN 0372-7025 (Print) ISSN 2571-113X (Online) DOI: 10.31482/mmsl.2019.008 Since 1925 REVIEW ARTICLE A SHORT HISTORY OF IMUREGEN – AN ORIGINAL TISSUE EXTRACT Klara Kubelkova and Ales Macela Department of Molecular Pathology and Biology, Faculty of Military Health Sciences, University of Defence, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic Received 14th March 2019. Accepted 29th April 2019. Published 6th September 2019. Summary Imuregen is a unique dietary supplement that was developed by leading Czech immunologists, made and distributed from the Czech Republic, and first came out in the 1950s. Imuregen boasts of decades of research and clinical exposure. It was created for the immune system sourced only from all natural ingredients that support cellular immunity and energy, tissue regeneration, wound healing, endocrine gland repair, intestinal integrity, memory enhancement, and has antitumor effects. Key words: tissue extract; biological response modulator; Imuregen; Retisin Introduction Natural biological products in the forms of teas or extracts were the first drugs for improving the fitness of human beings in ancient times. Over the course of the millennia, an immense amount of empirical experience has been ac- cumulated. As passed from generation to generation, this has given rise to numerous innovative therapies. Their usage continues up to the present day, especially in the forms of prebiotics, probiotics, and/or nutritional supplements to support regeneration or innate immune responsiveness during unspecific sicknesses or during convalescence from infection or cancerous illnesses. The U.S. National Library of Medicine defines the term “tissue extracts” as: “Preparations made from animal tissues or organs (animal structures). -
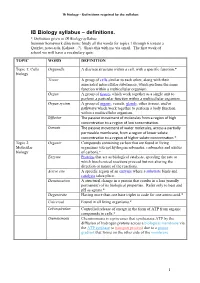
IB Biology Syllabus – Definitions
IB Biology – Definitions required by the syllabus IB Biology syllabus – definitions. * Definition given in IB Biology syllabus Summer homework directions: Study all the words for topics 1 through 6 (create a Quizlet, notecards, Kahoot…?). Share this with me via email. The first week of school we will have a vocabulary quiz. TOPIC WORD DEFINITION Topic 1: Cells Organelle A discrete structure within a cell, with a specific function.* biology Tissue A group of cells similar to each other, along with their associated intercellular substances, which perform the same function within a multicellular organism. Organ A group of tissues which work together as a single unit to perform a particular function within a multicellular organism. Organ system A group of organs, vessels, glands, other tissues, and/or pathways which work together to perform a body function within a multicellular organism. Diffusion The passive movement of molecules from a region of high concentration to a region of low concentration. Osmosis The passive movement of water molecules, across a partially permeable membrane, from a region of lower solute concentration to a region of higher solute concentration.* Topic 2: Organic Compounds containing carbon that are found in living Molecular organisms (except hydrogencarbonates, carbonates and oxides biology of carbon).* Enzyme Proteins that act as biological catalysts, speeding the rate at which biochemical reactions proceed but not altering the direction or nature of the reactions. Active site A specific region of an enzyme where a substrate binds and catalysis takes place. Denaturation A structural change in a protein that results in a loss (usually permanent) of its biological properties. -
CELL CULTURE BASICS Handbook
CELL CULTURE BASICS Cell Culture Basics Culture Cell www.invitrogen.com/cellculturebasics Handbook B-087243 0110 Contents Introduction . .1 Purpose of the Handbook . 1. Introduction to Cell Culture . 2. What is Cell Culture? . 2 Finite vs Continuous Cell Line . .2 Culture Conditions . .2 Cryopreservation . .2 Morphology of Cells in Culture . 3 Applications of Cell Culture . .3 Cell Culture Laboratory . .4 Safety . 4. Biosafety Levels . 4 SDS . 5 Safety Equipment . 5 Personal Protective Equipment (PPE) . 5 Safe Laboratory Practices . .5 Cell Culture Equipment . 6. Basic Equipment . 6 Expanded Equipment . .6 Additional Supplies . .6 Cell Culture Laboratory . 7. Aseptic Work Area . 7 Cell Culture Hood . .7 Cell Culture Hood Layout . .8 Incubator . 9 Storage . 9 Cryogenic Storage . .10 Cell Counter . .10 Cell Culture Basics | i Contents Aseptic Technique . .11 Introduction . .11 Sterile Work Area . .11 Good Personal Hygiene . .11 Sterile Reagents and Media . .12 Sterile Handling . .12 Aseptic Technique Checklist . .13 Biological Contamination . .14 Introduction . .14 Bacteria . .14 Yeasts . .15 Molds . .15 Viruses . .16 Mycoplasma . 16 Cross-Contamination . .17 Using Antibiotics . .17 Cell Culture Basics . .18 Cell Lines . .18 Selecting the Appropriate Cell Line . .18 Acquiring Cell Lines . 18 Culture Environment . .19 Adherent vs Suspension Culture . .19 Media . .20 pH . .21 CO2 . .21 Temperature . .21 Cell Morphology . .22 Mammalian Cells . .22 Variations in Mammalian Cell Morphology . .22 Morphology of 293 Cells . .23 Insect Cells . .24 Morphology of Sf21 Cells . .24 Morphology of Sf9 Cells . .25 ii | Cell Culture Basics Contents Methods . .26 Guidelines for Maintaining Cultured Cells . .26 What is Subculture? . .26 When to Subculture? . .27 Media Recommendations for Common Cell Lines .