An Introduction to Stem Cell Biology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. Introduction and Literature Review
1. Introduction and literature review 1.1 Introduction 1.1.1 Defintion of blood Blood is described as a specialized connective tissue,which circulates in a closed system of blood vessels. (Monica.C, 2009) 1.1.2 Blood components Plasma is 55% of the total blood ,plasma cosist of albumin,globulin,water,electrolyte and many other organic and inorganic substances. (Monica.C, 2009) Blood cells is 45% of total blood and encompass;White blood cells(WBCs),Red blood cells(RBCs),and Platelets(Plts). (Monica.C, 2009) 1.1.3 Function of blood -Respiration: transport of oxygen from the lung to tissues and carbon dioxide from tissues to the lungs. -Excreation:transport of metabolic waste to the lungs,kidneys,skin and intestine for removal. - Maintain of normal acid –base balance. -Nutrition of body . -Part of immune system. (Monica.C, 2009) 1 1.1.4 Haemopoiesis Is the general aspect of blood cells formation. (Monica.C, 2009) Haemopoiesis occurs at different anatomical sites the course of development from embryonic life to adult life this site is, up to 2 month of gestation.The haemopoiesis occurs in yolk sac of the embryo.This period called (Myeloblastic period). (Monica.C, 2009) 2-7 month of gestation, this period called (Haepatic period). (Monica.C, 2009) Only important site of all hemopoiesis site after birth,an exception is lymphocyte production which occur in other organ in addition to the bone marrow.This period called(Myeloid period). (Monica.C, 2009) 1.1.5 Development of haemopoiesis The general most commonly accepted view is that blood cells development from small population of stem cells. -

Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease Bone Marrow (Stem Cell) Transplant
Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease Bone Marrow (Stem Cell) Transplant for Sickle Cell Disease 1 Produced by St. Jude Children’s Research Hospital Departments of Hematology, Patient Education, and Biomedical Communications. Funds were provided by St. Jude Children’s Research Hospital, ALSAC, and a grant from the Plough Foundation. This document is not intended to take the place of the care and attention of your personal physician. Our goal is to promote active participation in your care and treatment by providing information and education. Questions about individual health concerns or specifi c treatment options should be discussed with your physician. For more general information on sickle cell disease, please visit our Web site at www.stjude.org/sicklecell. Copyright © 2009 St. Jude Children’s Research Hospital How did bone marrow (stem cell) transplants begin for children with sickle cell disease? Bone marrow (stem cell) transplants have been used for the treatment and cure of a variety of cancers, immune system diseases, and blood diseases for many years. Doctors in the United States and other countries have developed studies to treat children who have severe sickle cell disease with bone marrow (stem cell) transplants. How does a bone marrow (stem cell) transplant work? 2 In a person with sickle cell disease, the bone marrow produces red blood cells that contain hemoglobin S. This leads to the complications of sickle cell disease. • To prepare for a bone marrow (stem cell) transplant, strong medicines, called chemotherapy, are used to weaken or destroy the patient’s own bone marrow, stem cells, and infection fi ghting system. -

Tissue Tregs and Maintenance of Tissue Homeostasis
fcell-09-717903 August 12, 2021 Time: 13:32 # 1 REVIEW published: 18 August 2021 doi: 10.3389/fcell.2021.717903 Tissue Tregs and Maintenance of Tissue Homeostasis Qing Shao1,2,3,4†, Jian Gu1,2,3,4†, Jinren Zhou1,2,3,4†, Qi Wang1,2,3,4, Xiangyu Li1,2,3,4, Zhenhua Deng1,2,3,4 and Ling Lu1,2,3,4* 1 Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China, 2 Research Unit of Liver Transplantation and Transplant Immunology, Chinese Academy of Medical Sciences, Nanjing, China, 3 Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, Nanjing Medical University Affiliated Cancer Hospital, Nanjing, China, 4 Jiangsu Key Laboratory of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China Regulatory T cells (Tregs) specifically expressing Forkhead box P3 (Foxp3) play roles in suppressing the immune response and maintaining immune homeostasis. Edited by: After maturation in the thymus, Tregs leave the thymus and migrate to lymphoid Ivan Dzhagalov, tissues or non-lymphoid tissues. Increasing evidence indicates that Tregs with unique National Yang-Ming University, Taiwan characteristics also have significant effects on non-lymphoid peripheral tissues. Tissue- Reviewed by: resident Tregs, also called tissue Tregs, do not recirculate in the blood or lymphatics Dipayan Rudra, ImmunoBiome Inc., South Korea and attain a unique phenotype distinct from common Tregs in circulation. This review Ying Shao, first summarizes the phenotype, function, and cytokine expression of these Tregs in Temple University, United States visceral adipose tissue, skin, muscle, and other tissues. Then, how Tregs are generated, *Correspondence: Ling Lu home, and are attracted to and remain resident in the tissue are discussed. -

Introduction to the Cell Cell History Cell Structures and Functions
Introduction to the cell cell history cell structures and functions CK-12 Foundation December 16, 2009 CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and distribution of high quality educational content that will serve both as core text as well as provide an adaptive environment for learning. Copyright ©2009 CK-12 Foundation This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License. To view a copy of this license, visit http://creativecommons.org/licenses/by-sa/3.0/us/ or send a letter to Creative Commons, 171 Second Street, Suite 300, San Francisco, California, 94105, USA. Contents 1 Cell structure and function dec 16 5 1.1 Lesson 3.1: Introduction to Cells .................................. 5 3 www.ck12.org www.ck12.org 4 Chapter 1 Cell structure and function dec 16 1.1 Lesson 3.1: Introduction to Cells Lesson Objectives • Identify the scientists that first observed cells. • Outline the importance of microscopes in the discovery of cells. • Summarize what the cell theory proposes. • Identify the limitations on cell size. • Identify the four parts common to all cells. • Compare prokaryotic and eukaryotic cells. Introduction Knowing the make up of cells and how cells work is necessary to all of the biological sciences. Learning about the similarities and differences between cell types is particularly important to the fields of cell biology and molecular biology. -

Characterization of Embryonic Stem Cell-Differentiated Cells As Mesenchymal Stem Cells
The University of Southern Mississippi The Aquila Digital Community Honors Theses Honors College Fall 12-2015 Characterization of Embryonic Stem Cell-Differentiated Cells as Mesenchymal Stem Cells Rachael N. Kuehn University of Southern Mississippi Follow this and additional works at: https://aquila.usm.edu/honors_theses Part of the Cell Biology Commons Recommended Citation Kuehn, Rachael N., "Characterization of Embryonic Stem Cell-Differentiated Cells as Mesenchymal Stem Cells" (2015). Honors Theses. 349. https://aquila.usm.edu/honors_theses/349 This Honors College Thesis is brought to you for free and open access by the Honors College at The Aquila Digital Community. It has been accepted for inclusion in Honors Theses by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. The University of Southern Mississippi Characterization of Embryonic Stem Cell-Differentiated Cells as Mesenchymal Stem Cells by Rachael Nicole Kuehn A Thesis Submitted to the Honors College of The University of Southern Mississippi in Partial Fulfillment of the Requirements for the Degree of Bachelor of Science in the Department of Biological Sciences December 2015 ii Approved by ______________________________ Yanlin Guo, Ph.D., Thesis Adviser Professor of Biological Sciences ______________________________ Shiao Y. Wang, Ph.D., Chair Department of Biological Sciences ______________________________ Ellen Weinauer, Ph.D., Dean Honors College iii ABSTRACT Embryonic stem cells (ESCs), due to their ability to differentiate into different cell types while still maintaining a high proliferation capacity, have been considered as a potential cell source in regenerative medicine. However, current ESC differentiation methods are low yielding and create heterogeneous cell populations. -

The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models
biomolecules Review The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models Meera Krishnan 1, Sahil Kumar 1, Luis Johnson Kangale 2,3 , Eric Ghigo 3,4 and Prasad Abnave 1,* 1 Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone, Gurgaon-Faridabad Ex-pressway, Faridabad 121001, India; [email protected] (M.K.); [email protected] (S.K.) 2 IRD, AP-HM, SSA, VITROME, Aix-Marseille University, 13385 Marseille, France; [email protected] 3 Institut Hospitalo Universitaire Méditerranée Infection, 13385 Marseille, France; [email protected] 4 TechnoJouvence, 13385 Marseille, France * Correspondence: [email protected] Abstract: Adult stem cells (ASCs) are the undifferentiated cells that possess self-renewal and differ- entiation abilities. They are present in all major organ systems of the body and are uniquely reserved there during development for tissue maintenance during homeostasis, injury, and infection. They do so by promptly modulating the dynamics of proliferation, differentiation, survival, and migration. Any imbalance in these processes may result in regeneration failure or developing cancer. Hence, the dynamics of these various behaviors of ASCs need to always be precisely controlled. Several genetic and epigenetic factors have been demonstrated to be involved in tightly regulating the proliferation, differentiation, and self-renewal of ASCs. Understanding these mechanisms is of great importance, given the role of stem cells in regenerative medicine. Investigations on various animal models have played a significant part in enriching our knowledge and giving In Vivo in-sight into such ASCs regulatory mechanisms. In this review, we have discussed the recent In Vivo studies demonstrating the role of various genetic factors in regulating dynamics of different ASCs viz. -

Boosting the Cellular Potency of Embryonic Stem Cells by Spliceosome Targeting ✉ Wilfried A
Signal Transduction and Targeted Therapy www.nature.com/sigtrans RESEARCH HIGHLIGHT OPEN Boosting the cellular potency of embryonic stem cells by spliceosome targeting ✉ Wilfried A. Kues1 Signal Transduction and Targeted Therapy (2021) 6:324; https://doi.org/10.1038/s41392-021-00743-9 In recent work published in Cell, Shen et al.1 identified transfected ES cells with short interfering RNAs against different spliceosome inhibition in embryonic stem (ES) cells as a key spliceosome transcripts (the spliceosome consisted of 5 core and mechanism for the transition from pluri- to totipotency. Spliceo- several cofactor subunits, here 14 transcripts were targeted), some inhibition, achieved by RNA interference or the chemical respectively. Transient repression of 10 of the 14 splicing factors inhibitor pladienolide B, may gain widespread relevance to the resulted in ES cells, which maintained the typical colony culture of totipotent ES cells, in vitro differentiation of extra- morphology, however, pluripotent marker genes—Oct4 (Pou5f1), embryonal tissue and organoids, translation to the maintenance of Nanog, Sox2, Zfp42 and others—became down-regulated, at the pluripotent cells of other mammal species, including humans, and same time marker genes of totipotency—particularly Zscan4s and a better molecular understanding of cellular potency in stem cells MERVL—were up-regulated. Zscan4s (Zink finger and SCAN and cancer. domain containing 4) is a transcription factor and MERVL (murine The first successful isolation and maintenance of ES derived endogenous retrovirus L) an endogenous retrovirus with a usually fi 1234567890();,: from the inner cell mass (ICM) of murine blastocyst stages was restricted expression to 2-cell embryos. These results were veri ed described in 1981,2 and since then acted as game changer for by supplementing the culture medium with pladienolide B, a genetic studies in this mammalian model organism. -

Epigenetic Metabolites License Stem Cell States
CHAPTER SIX Epigenetic metabolites license stem cell states Logeshwaran Somasundarama,b,†, Shiri Levya,b,†, Abdiasis M. Husseina,b, Devon D. Ehnesa,b, Julie Mathieub,c, Hannele Ruohola-Bakera,b,∗ aDepartment of Biochemistry, University of Washington, Seattle, WA, United States bInstitute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, WA, United States cDepartment of Comparative Medicine, University of Washington, Seattle, WA, United States ∗ Corresponding author: e-mail address: [email protected] Contents 1. Introduction 210 2. Stem cell energetics 210 3. Metabolism of quiescent stem cells 212 3.1 Adult stem cells 212 3.2 Pluripotent stem cell quiescence, diapause 216 4. Metabolism of active stem cells 217 4.1 Metabolism after fertilization 217 4.2 Metabolism of pre-implantation and post-implantation pluripotent stem cells 218 4.3 Metabolism of actively cycling adult stem cells: MSC as case-study 220 5. HIF, the master regulator of metabolism 222 6. Epigenetic signatures and epigenetic metabolites 224 6.1 Epigenetic signatures of naïve and primed pluripotent stem cells 224 6.2 Epigenetic signatures of adult stem cells 227 6.3 Epigenetic metabolites 228 7. Conclusion 229 Acknowledgments 230 References 230 Further reading 240 Abstract It has become clear during recent years that stem cells undergo metabolic remodeling during their activation process. While these metabolic switches take place in pluripotency as well as adult stem cell populations, the rules that govern the switch are not clear. † Equal contribution. # Current Topics in Developmental Biology, Volume 138 2020 Elsevier Inc. 209 ISSN 0070-2153 All rights reserved. https://doi.org/10.1016/bs.ctdb.2020.02.003 210 Logeshwaran Somasundaram et al. -
Creating a Census of Human Cells
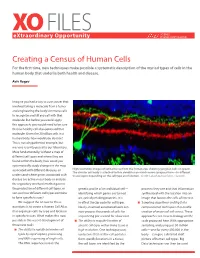
XO FILES SCIENCE eXtraordinary Opportunity PHILANTHROPY ALLIANCE Creating a Census of Human Cells For the first time, new techniques make possible a systematic description of the myriad types of cells in the human body that underlie both health and disease. Aviv Regev Imagine you had a way to cure cancer that involved taking a molecule from a tumor and engineering the body’s immune cells to recognize and kill any cell with that molecule. But before you could apply this approach, you would need to be sure that no healthy cell also expressed that molecule. Given the 20 trillion cells in a human body, how would you do that? This is not a hypothetical example, but was one recently posed to our laboratory. More fundamentally, without a map of different cell types and where they are found within the body, how could you systematically study changes in the map associated with different diseases, or High resolution images of retinal tissue from the human eye, showing ganglion cells (in green). The circular cell body is attached to thin dendrites on which nerve synapses form—in different understand where genes associated with tissue layers depending on the cell type and function. Credit: Lab of Joshua Sanes, Harvard. disease are active in our body or analyze the regulatory mechanisms that govern the production of different cell types, or genetic profile of an individual cell— proteins they use and that information sort out how different cell types combine identifying which genes are turned synthesized with the location into an to form specific tissues? on, actively making proteins. -

Genetic Manipulation of Stem Cells Eleni Papanikolaou1,2*, Kalliopi I
logy & Ob o st ec e tr n i y c s G Papanikolaou et al. Gynecol Obstetric 2011, S:6 Gynecology & Obstetrics DOI: 10.4172/2161-0932.S6-001 ISSN: 2161-0932 Review Article Open Access Genetic Manipulation of Stem Cells Eleni Papanikolaou1,2*, Kalliopi I. Pappa1,3 and Nicholas P. Anagnou1,2 1Laboratory of Cell and Gene Therapy, Centre for Basic Research, Biomedical Research Foundation of the Academy of Athens (BRFAA), Athens, Greece 2Laboratory of Biology, University of Athens School of Medicine, Athens, Greece 3First Department of Obstetrics and Gynecology, University of Athens School of Medicine, Alexandra Hospital, Athens, Greece Abstract Stem cells have the remarkable potential for self-renewal and differentiation into many cell types in the body during early life and development. In addition, in many tissues they constitute a source of internal repair system, dividing essentially without limit to replenish damaged or dead cells. After division, each new cell has the potential either to retain the stem cell status or to differentiate to a more specialized cell type, such as a red blood cell, a brain cell or a heart cell. Until recently, three types of stem cells from animals and humans have been characterized, i.e. embryonic stem cells, fetal stem cells and somatic adult stem cells. However, in late 2007, researchers accomplished another breakthrough by identifying conditions that allow some specialized adult cells to be “reprogrammed” genetically to assume a stem cell-like state. These cells, called induced pluripotent stem cells (iPSCs), express genes and factors important for maintaining the unique properties and features of embryonic stem cells. -

Genetics and Stem Cell Research A.Genetics
7: Genetics and Stem Cell Research A.Genetics 1. Introduction The principal special feature of genetics research is that the result of the study applies not only to the proband but also influences her lineage both in the past and in the future. For example genetic studies demonstrated Thomas Jefferson’s sexual relationship with his slave Sally Hemings and defined their descendants to this day. As we all know from television, genetic studies can be done from any tissue fragment that contains DNA so that studies of surgical specimens, biopsy materials, hair, epithelium and blood samples can all be utilized for extensive genetic studies. 2. Sampling Some DNA is more medically valuable than other. Samples from isolated populations in which a particular disorder is prevalent have a much greater probability of yielding the causal gene(s) because they have fewer genome variations than in the general population. Once isolated, the genetic material associated with the disorder has a good chance of yielding novel diagnostic and/or therapeutic approaches for the disorder. 3. Property rights A persistent question is whether the providers of the genetic material have any rights to the products created from their genetic material. These days, most consent forms are written explicitly to exclude intellectual property rights from the subjects. As might be imagined, this smacks of exploitation in the developing world. Negotiation of a monetary return to the community has sometimes been concluded. Important and lucrative products have been derived from individuals’ genomes without their receiving royalties or other compensation. However, the knowledge, technical expertise, and capital needed to make a useful product from a blood or tissue sample come from the company not the donor. -

Robustness and Timing of Cellular Differentiation Through Population-Based Symmetry Breaking
AR TI CLE Robustness AND TIMING OF CELLULAR DIFFERENTIATION THROUGH population-based SYMMETRY BREAKING Angel StanoeV1 | Christian Schröter1 | Aneta KOSESKA1∗ 1Department OF Systemic Cell Biology, Max Planck Institute OF Molecular PhYSIOLOGY, Although COORDINATED BEHAVIOR OF MULTICELLULAR ORGANISMS Dortmund, GermanY RELIES ON INTERCELLULAR communication, THE DYNAMICAL mech- Correspondence ANISM THAT UNDERLIES SYMMETRY BREAKING IN HOMOGENEOUS Email: populations, THEREBY GIVING RISE TO HETEROGENEOUS CELL TYPES ∗[email protected] REMAINS UNCLEAR. In CONTRAST TO THE PREVALENT cell-intrinsic VIEW OF DIFFERENTIATION WHERE MULTISTABILITY ON THE SINGLE CELL LEVEL IS A NECESSARY pre-requisite, WE PROPOSE THAT het- EROGENEOUS CELLULAR IDENTITIES EMERGE AND ARE MAINTAINED COOPERATIVELY ON A POPULATION LEVel. Identifying A GENERIC SYMMETRY BREAKING mechanism, WE DEMONSTRATE THAT ROBUST PROPORTIONS BETWEEN DIFFERENTIATED CELL TYPES ARE INTRINSIC FEATURES OF THIS INHOMOGENEOUS STEADY STATE solution. Crit- ICAL ORGANIZATION IN THE VICINITY OF THE SYMMETRY BREAKING BIFURCATION ON THE OTHER hand, SUGGESTS THAT TIMING OF cel- LULAR DIFFERENTIATION EMERGES FOR GROWING POPULATIONS IN A self-organized MANNER. Setting A CLEAR DISTINCTION BETWEEN pre-eXISTING ASYMMETRIES AND SYMMETRY BREAKING EVents, WE THUS DEMONSTRATE THAT EMERGENT SYMMETRY breaking, IN CONJUNCTION WITH ORGANIZATION NEAR CRITICALITY NECESSARILY LEADS TO ROBUSTNESS AND ACCURACY DURING DEVelopment. KEYWORDS SYMMETRY breaking; differentiation; INHOMOGENEOUS STEADY STATE Abbreviations: