TRPV2 Is Critical for the Maintenance of Cardiac Structure and Function in Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -
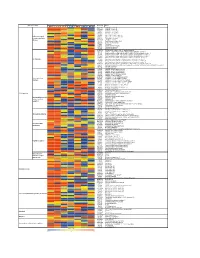
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Investigational Drugs in Early Phase Clinical Trials Targeting Thermotransient Receptor Potential (Thermotrp) Channels
Expert Opinion on Investigational Drugs ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/ieid20 Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels Asia Fernández-Carvajal , Rosario González-Muñiz , Gregorio Fernández- Ballester & Antonio Ferrer-Montiel To cite this article: Asia Fernández-Carvajal , Rosario González-Muñiz , Gregorio Fernández- Ballester & Antonio Ferrer-Montiel (2020): Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels, Expert Opinion on Investigational Drugs, DOI: 10.1080/13543784.2020.1825680 To link to this article: https://doi.org/10.1080/13543784.2020.1825680 Published online: 29 Sep 2020. Submit your article to this journal Article views: 31 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ieid20 EXPERT OPINION ON INVESTIGATIONAL DRUGS https://doi.org/10.1080/13543784.2020.1825680 REVIEW Investigational drugs in early phase clinical trials targeting thermotransient receptor potential (thermoTRP) channels Asia Fernández-Carvajala, Rosario González-Muñizb, Gregorio Fernández-Ballestera and Antonio Ferrer-Montiela aInstituto De Investigación, Desarrollo E Innovación En Biotecnología Sanitaria De Elche (Idibe), Universitas Miguel Hernández, Alicante, Spain; bInstituto De Química Médica, CSIC, Madrid, Spain ABSTRACT ARTICLE HISTORY Introduction: Thermo transient receptor potential (thermoTRP) channels are some of the most inten Received 15 June 2020 sely pursued therapeutic targets of the past decade. They are considered promising targets of numer Accepted 15 September ous diseases including chronic pain and cancer. Modulators of these proteins, in particular TRPV1-4, 2020 TRPM8 and TRPA1, have reached clinical development, but none has been approved for clinical practice KEYWORDS yet. -

Snapshot: Mammalian TRP Channels David E
SnapShot: Mammalian TRP Channels David E. Clapham HHMI, Children’s Hospital, Department of Neurobiology, Harvard Medical School, Boston, MA 02115, USA TRP Activators Inhibitors Putative Interacting Proteins Proposed Functions Activation potentiated by PLC pathways Gd, La TRPC4, TRPC5, calmodulin, TRPC3, Homodimer is a purported stretch-sensitive ion channel; form C1 TRPP1, IP3Rs, caveolin-1, PMCA heteromeric ion channels with TRPC4 or TRPC5 in neurons -/- Pheromone receptor mechanism? Calmodulin, IP3R3, Enkurin, TRPC6 TRPC2 mice respond abnormally to urine-based olfactory C2 cues; pheromone sensing 2+ Diacylglycerol, [Ca ]I, activation potentiated BTP2, flufenamate, Gd, La TRPC1, calmodulin, PLCβ, PLCγ, IP3R, Potential role in vasoregulation and airway regulation C3 by PLC pathways RyR, SERCA, caveolin-1, αSNAP, NCX1 La (100 µM), calmidazolium, activation [Ca2+] , 2-APB, niflumic acid, TRPC1, TRPC5, calmodulin, PLCβ, TRPC4-/- mice have abnormalities in endothelial-based vessel C4 i potentiated by PLC pathways DIDS, La (mM) NHERF1, IP3R permeability La (100 µM), activation potentiated by PLC 2-APB, flufenamate, La (mM) TRPC1, TRPC4, calmodulin, PLCβ, No phenotype yet reported in TRPC5-/- mice; potentially C5 pathways, nitric oxide NHERF1/2, ZO-1, IP3R regulates growth cones and neurite extension 2+ Diacylglycerol, [Ca ]I, 20-HETE, activation 2-APB, amiloride, Cd, La, Gd Calmodulin, TRPC3, TRPC7, FKBP12 Missense mutation in human focal segmental glomerulo- C6 potentiated by PLC pathways sclerosis (FSGS); abnormal vasoregulation in TRPC6-/- -

Ca Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart
Journal of Cardiovascular Development and Disease Review Ca2+ Signaling in Cardiac Fibroblasts and Fibrosis-Associated Heart Diseases Jianlin Feng 1, Maria K. Armillei 1, Albert S. Yu 1, Bruce T. Liang 1, Loren W. Runnels 2,* and Lixia Yue 1,* 1 Calhoun Cardiology Center, Department of Cell Biology, University of Connecticut Health Center, Farmington, CT 06030, USA; [email protected] (J.F.); [email protected] (M.K.A.); [email protected] (A.S.Y.); [email protected] (B.T.L.) 2 Department of Pharmacology, Rutgers, Robert Wood Johnson Medical School, Piscataway, NJ 08854, USA * Correspondence: [email protected] (L.W.R.); [email protected] (L.Y.) Received: 11 August 2019; Accepted: 18 September 2019; Published: 23 September 2019 Abstract: Cardiac fibrosis is the excessive deposition of extracellular matrix proteins by cardiac fibroblasts and myofibroblasts, and is a hallmark feature of most heart diseases, including arrhythmia, hypertrophy, and heart failure. This maladaptive process occurs in response to a variety of stimuli, including myocardial injury, inflammation, and mechanical overload. There are multiple signaling pathways and various cell types that influence the fibrogenesis cascade. Fibroblasts and myofibroblasts are central effectors. Although it is clear that Ca2+ signaling plays a vital role in this pathological process, what contributes to Ca2+ signaling in fibroblasts and myofibroblasts is still not wholly understood, chiefly because of the large and diverse number of receptors, transporters, and ion channels that influence intracellular Ca2+ signaling. Intracellular Ca2+ signals are generated by Ca2+ release from intracellular Ca2+ stores and by Ca2+ entry through a multitude of Ca2+-permeable ion channels in the plasma membrane. -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy
International Journal of Molecular Sciences Review An Update on Connexin Gap Junction and Hemichannels in Diabetic Retinopathy Jorge González-Casanova 1 , Oliver Schmachtenberg 2, Agustín D. Martínez 3, Helmuth A. Sanchez 3, Paloma A. Harcha 3 and Diana Rojas-Gomez 4,* 1 Instituto de Ciencias Biomédicas, Facultad de Ciencias de la Salud, Universidad Autónoma de Chile, Santiago 8910060, Chile; [email protected] 2 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Biología, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] 3 Centro Interdisciplinario de Neurociencia de Valparaíso, Instituto de Neurociencia, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso 2360102, Chile; [email protected] (A.D.M.); [email protected] (H.A.S.); [email protected] (P.A.H.) 4 Escuela de Nutrición y Dietética, Facultad de Medicina, Universidad Andres Bello, Santiago 8370146, Chile * Correspondence: [email protected]; Tel.: +56-2-26618559 Abstract: Diabetic retinopathy (DR) is one of the main causes of vision loss in the working age popu- lation. It is characterized by a progressive deterioration of the retinal microvasculature, caused by long-term metabolic alterations inherent to diabetes, leading to a progressive loss of retinal integrity and function. The mammalian retina presents an orderly layered structure that executes initial but complex visual processing and analysis. Gap junction channels (GJC) forming electrical synapses are present in each retinal layer and contribute to the communication between different cell types. Citation: González-Casanova, J.; In addition, connexin hemichannels (HCs) have emerged as relevant players that influence diverse Schmachtenberg, O.; Martínez, A.D.; physiological and pathological processes in the retina. -

Conserved Allosteric Pathways for Activation of TRPV3 Revealed Through Engineering Vanilloid-Sensitivity Feng Zhang1,2*, Kenton Jon Swartz1, Andres Jara-Oseguera1*
RESEARCH ARTICLE Conserved allosteric pathways for activation of TRPV3 revealed through engineering vanilloid-sensitivity Feng Zhang1,2*, Kenton Jon Swartz1, Andres Jara-Oseguera1* 1Molecular Physiology and Biophysics Section, Porter Neuroscience Research Center, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, United States; 2Department of Biochemistry, University of Utah, Salt Lake City, United States Abstract The Transient Receptor Potential Vanilloid 1 (TRPV) channel is activated by an array of stimuli, including heat and vanilloid compounds. The TRPV1 homologues TRPV2 and TRPV3 are also activated by heat, but sensitivity to vanilloids and many other agonists is not conserved among TRPV subfamily members. It was recently discovered that four mutations in TRPV2 are sufficient to render the channel sensitive to the TRPV1-specific vanilloid agonist resiniferatoxin (RTx). Here, we show that mutation of six residues in TRPV3 corresponding to the vanilloid site in TRPV1 is sufficient to engineer RTx binding. However, robust activation of TRPV3 by RTx requires facilitation of channel opening by introducing mutations in the pore, temperatures > 30˚C, or sensitization with another agonist. Our results demonstrate that the energetics of channel activation can determine the apparent sensitivity to a stimulus and suggest that allosteric pathways for activation are conserved in the TRPV family. DOI: https://doi.org/10.7554/eLife.42756.001 *For correspondence: [email protected] (FZ); Introduction [email protected] (AJ) Transient receptor potential (TRP) cation channels are involved in a diverse array of physiological functions (Li, 2017), with many of them acting as sensory detectors of stimuli such as temperature, Competing interest: See natural products and various cell-signaling molecules (Flockerzi, 2007). -

The Ca2+-Permeable Cation Transient Receptor Potential
1521-0111/92/3/193–200$25.00 https://doi.org/10.1124/mol.116.107946 MOLECULAR PHARMACOLOGY Mol Pharmacol 92:193–200, September 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics MINIREVIEW—MOLECULAR PHARMACOLOGY IN CHINA The Ca21-Permeable Cation Transient Receptor Potential TRPV3 Channel: An Emerging Pivotal Target for Itch and Skin Diseases Gongxin Wang and KeWei Wang Department of Pharmacology, Qingdao University School of Pharmacy and Institute of Innovative Drugs, Qingdao University, Downloaded from Qingdao, Shandong Province, China Received December 20, 2016; accepted March 31, 2017 ABSTRACT Temperature-sensitive transient receptor potential (TRP) chan- syndrome, which is characterized by severe itching and molpharm.aspetjournals.org nels such as TRPA1 and TRPV1 have been identified as down- palmoplantar and periorificial keratoderma, unveils its crucial stream ion channel targets in the transduction of itch. As a member role in chronic itch and skin diseases. In this review, we will of the temperature-sensitive TRP family, the Ca21-permeable focus on recent progress made in the understanding of nonselective cation channel TRPV3 is expressed abundantly TRPV3 that emerges as an attractive target for developing in skin keratinocytes. Recent identification of gain-of-function effective antipruritic therapy for chronic itch or skin-related mutations of human TRPV3 from patients with Olmsted diseases. attractive target for developing antipruritic therapy in chronic Introduction at ASPET Journals on September 28, 2021 itch or skin-related diseases. Itch (also known as pruritus) is an unpleasant sensation of The superfamily of TRP channels is composed of 28 mam- the skin, provoking the desire or reflex to scratch. -

An Aquaporin-4/Transient Receptor Potential Vanilloid 4 (AQP4/TRPV4) Complex Is Essential for Cell-Volume Control in Astrocytes
An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes Valentina Benfenatia,1, Marco Caprinib,1, Melania Doviziob, Maria N. Mylonakouc, Stefano Ferronib, Ole P. Ottersenc, and Mahmood Amiry-Moghaddamc,2 aInstitute for Nanostructured Materials, Consiglio Nazionale delle Ricerche, 40129 Bologna, Italy; bDepartment of Human and General Physiology, University of Bologna, 40127 Bologna, Italy; and cCenter for Molecular Biology and Neuroscience and Department of Anatomy, University of Oslo, 0317 Oslo, Norway Edited* by Peter Agre, Johns Hopkins Malaria Research Institute, Baltimore, MD, and approved December 27, 2010 (received for review September 1, 2010) Regulatory volume decrease (RVD) is a key mechanism for volume channels (VRAC). Osmolyte efflux through VRAC is thought to control that serves to prevent detrimental swelling in response to provide the driving force for water exit (6, 7). The factors that hypo-osmotic stress. The molecular basis of RVD is not understood. initiate RVD have received comparatively little attention. Here Here we show that a complex containing aquaporin-4 (AQP4) and we test our hypothesis that activation of astroglial RVD depends transient receptor potential vanilloid 4 (TRPV4) is essential for RVD on a molecular interaction between AQP4 and TRPV4. fi in astrocytes. Astrocytes from AQP4-KO mice and astrocytes treated Our hypothesis rests on our recent nding that TRPV4 is with TRPV4 siRNA fail to respond to hypotonic stress by increased strongly expressed in astrocytic endfeet membranes abutting the – intracellular Ca2+ and RVD. Coimmunoprecipitation and immunohis- pia (including the extension of the pia that lines the Virchow tochemistry analyses show that AQP4 and TRPV4 interact and coloc- Robin spaces) and in endfeet underlying ependyma of the ven- alize. -

TRPV2 Channel As a Possible Drug Target for the Treatment of Heart Failure
Laboratory Investigation (2020) 100:207–217 https://doi.org/10.1038/s41374-019-0349-z REVIEW ARTICLE TRPV2 channel as a possible drug target for the treatment of heart failure 1 1 2 1 Yuko Iwata ● Shin Ito ● Shigeo Wakabayashi ● Masafumi Kitakaze Received: 29 September 2019 / Revised: 9 November 2019 / Accepted: 13 November 2019 / Published online: 19 December 2019 © The Author(s), under exclusive licence to United States and Canadian Academy of Pathology 2019 Abstract Heart transplantation is currently the only viable option available for the treatment of severe heart failure conditions such as dilated cardiomyopathy. Hence, novel drugs for treating such conditions need to be developed urgently. Recent studies suggest that Ca2+ overload is involved in the onset and progression of dilated cardiomyopathy, and thus heart failure. The expression and activation of the Ca2+ permeable channel, transient receptor potential vanilloid 2 (TRPV2) channel have been found to play an essential role in sustained intracellular Ca2+ concentration increase, leading to heart failure. However, since there have been no TRPV2-specific inhibitors available until recently, the effect of TRPV2 inhibition on the pathology has not been clearly elucidated. Recent reports show that inhibiting TRPV2 activity effectively improves cardiac function, fi 1234567890();,: 1234567890();,: suppressing myocardial brosis and ameliorating the prognosis in animal models of cardiomyopathy with heart failure. In addition to that, inflammation is reported to be involved in the development of heart failure. Here, we review the recent findings on TRPV2 in cardiomyocytes and immune cells involved in the development of heart failure and discuss the current progress of drug development for the treatment of heart failure via targeting TRPV2.